Climbing fibers provide essential instructive signals for associative learning
- PMID: 38565684
- PMCID: PMC11088996
- DOI: 10.1038/s41593-024-01594-7
Climbing fibers provide essential instructive signals for associative learning
Abstract
Supervised learning depends on instructive signals that shape the output of neural circuits to support learned changes in behavior. Climbing fiber (CF) inputs to the cerebellar cortex represent one of the strongest candidates in the vertebrate brain for conveying neural instructive signals. However, recent studies have shown that Purkinje cell stimulation can also drive cerebellar learning and the relative importance of these two neuron types in providing instructive signals for cerebellum-dependent behaviors remains unresolved. In the present study we used cell-type-specific perturbations of various cerebellar circuit elements to systematically evaluate their contributions to delay eyeblink conditioning in mice. Our findings reveal that, although optogenetic stimulation of either CFs or Purkinje cells can drive learning under some conditions, even subtle reductions in CF signaling completely block learning to natural stimuli. We conclude that CFs and corresponding Purkinje cell complex spike events provide essential instructive signals for associative cerebellar learning.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


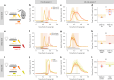
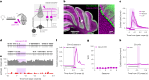
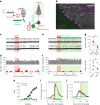
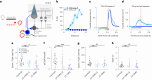



References
-
- Albus JS. A theory of cerebellar function. Math. Biosci. 1971;10:25–61.
-
- Ito M. Neural design of the cerebellar motor control system. Brain Res. 1972;40:81–84. - PubMed
-
- Raymond JL, Lisberger SG, Mauk MD. The cerebellum: a neuronal learning machine? Science. 1996;272:1126–1131. - PubMed
-
- Mauk MD, Donegan NH. A model of Pavlovian eyelid conditioning based on the synaptic organization of the cerebellum. Learn. Mem. 1997;4:130–158. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

