Emerging racial disparities among Medicare beneficiaries and Veterans with metastatic castration-sensitive prostate cancer
- PMID: 38565911
- PMCID: PMC11543599
- DOI: 10.1038/s41391-024-00815-1
Emerging racial disparities among Medicare beneficiaries and Veterans with metastatic castration-sensitive prostate cancer
Abstract
Background: Previous studies have shown that Black men receive worse prostate cancer care than White men. This has not been explored in metastatic castration-sensitive prostate cancer (mCSPC) in the current treatment era.
Methods: We evaluated treatment intensification (TI) and overall survival (OS) in Medicare (2015-2018) and Veterans Health Administration (VHA; 2015-2019) patients with mCSPC, classifying first-line mCSPC treatment as androgen deprivation therapy (ADT) + novel hormonal therapy; ADT + docetaxel; ADT + first-generation nonsteroidal antiandrogen; or ADT alone.
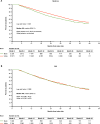
Results: We analyzed 2226 Black and 16,071 White Medicare, and 1020 Black and 2364 White VHA patients. TI was significantly lower for Black vs White Medicare patients overall (adjusted odds ratio [OR] 0.68; 95% confidence interval [CI] 0.58-0.81) and without Medicaid (adjusted OR 0.70; 95% CI 0.57-0.87). Medicaid patients had less TI irrespective of race. OS was worse for Black vs White Medicare patients overall (adjusted hazard ratio [HR] 1.20; 95% CI 1.09-1.31) and without Medicaid (adjusted HR 1.13; 95% CI 1.01-1.27). OS was worse in Medicaid vs without Medicaid, with no significant OS difference between races. TI was significantly lower for Black vs White VHA patients (adjusted OR 0.75; 95% CI 0.61-0.92), with no significant OS difference between races.
Conclusions: Guideline-recommended TI was low for all patients with mCSPC, with less TI in Black patients in both Medicare and the VHA. Black race was associated with worse OS in Medicare but not the VHA. Medicaid patients had less TI and worse OS than those without Medicaid, suggesting poverty and race are associated with care and outcomes.
© 2024. The Author(s).
Conflict of interest statement
DJG reports other from American Association for Cancer Research; grants and personal fees from Astellas Pharma Inc.; personal fees from AstraZeneca; personal fees from Axess Oncology; grants, personal fees, and non-financial support from Bayer H/C Pharmaceuticals; grants and personal fees from BMS; grants from Calithera; grants from Capio Biosciences; grants from EMD Serono; grants, personal fees, and non-financial support from Exelixis Inc; personal fees from Flatiron; grants and personal fees from Janssen Pharmaceuticals; personal fees from Merck, Sharp & Dohme; personal fees from Michael J Hennessey Assoc; personal fees from Millennium Medical Publishing; personal fees from Myovant Sciences Inc.; personal fees from NCI Genitourinary; grants and personal fees from Novartis; personal fees from Physician Education Resource; personal fees from Propella Therapeutics; grants and personal fees from Pfizer Inc.; personal fees from RevHealth; grants, personal fees, and non-financial support from Sanofi; personal fees from Seattle Genetics; personal fees from UroGPO; personal fees and non-financial support from UroToday; personal fees from WebMD; personal fees from Xcures. NA reports no personal conflicts of interest since April 15, 2021; lifetime conflicts of interest include consultancy to Astellas Pharma Inc., Astra Zeneca, Aveo, Bayer, Bristol Myers Squibb, Calithera, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Gilead, Janssen, Merck, MEI Pharma, Nektar, Novartis, Pfizer Inc., Pharmacyclics, and Seattle Genetics; research funding to institution from Arnivas, Astellas Pharma Inc., Astra Zeneca, Bavarian Nordic, Bayer, Bristol Myers Squibb, Calithera, Celldex, Clovis, Crispr, Eisai, Eli Lilly, EMD Serono, Exelixis, Genentech, Gilead, Glaxo Smith Kline, Immunomedics, Janssen, Lava, Medivation, Merck, Nektar, Neoleukin, New Link Genetics, Novartis, Oric, Pfizer Inc., Prometheus, Rexahn, Roche, Sanofi, Seattle Genetics, Takeda, and Tracon. KR, DR, and RS own stocks or stock options and are employees of Pfizer Inc., a study sponsor. ZK reports serving on advisory boards for Bayer, Astellas Pharma Inc., Myovant, and Pfizer; consulting to Sesen Bio; and serving on a speakers’ bureau for Lantheus. RLB reports no disclosures. BE is an employee of Pfizer Inc., a study sponsor. HY, WS, and WG are employees of Analysis Group, which was a paid consultant to the sponsors in connection with the development of this manuscript. YL is co-owner of BRAVO4HEALTH LLC. AH is an employee of Pfizer Inc., a study sponsor; was an employee of Astellas Pharma Inc., a study sponsor, at the time of the study; and owns stock in Veru, Revance, and Insmed. SJF reports consulting to Pfizer Inc., Astellas Pharma Inc., Janssen, Dendreon, Sanofi, Myovant, Merck, AstraZeneca, Clovis, and Bayer. No other disclosures were reported.
Figures

Similar articles
-
Racial Differences in Survival and Healthcare Resource Utilization Among Medicaid-Insured Adults With Metastatic Prostate Cancer.Clin Genitourin Cancer. 2025 Apr;23(2):102291. doi: 10.1016/j.clgc.2024.102291. Epub 2024 Dec 12. Clin Genitourin Cancer. 2025. PMID: 39813906
-
Plain language summary: does race or income status affect the cancer treatments that patients with metastatic castration-sensitive prostate cancer (mCSPC) receive in the United States?Future Oncol. 2025 May;21(11):1307-1315. doi: 10.1080/14796694.2025.2485763. Epub 2025 Apr 25. Future Oncol. 2025. PMID: 40277241 Free PMC article.
-
Treatment intensification in metastatic castration-sensitive prostate cancer: a real-world study in Alberta, Canada.Future Oncol. 2025 Apr;21(10):1197-1207. doi: 10.1080/14796694.2025.2479374. Epub 2025 Mar 24. Future Oncol. 2025. PMID: 40126447 Free PMC article.
-
Current Systemic Therapy in Men with Metastatic Castration-Sensitive Prostate Cancer.Curr Oncol Rep. 2024 May;26(5):488-495. doi: 10.1007/s11912-024-01509-6. Epub 2024 Apr 9. Curr Oncol Rep. 2024. PMID: 38592590 Review.
-
First-line systemic therapy for metastatic castration-sensitive prostate cancer: An updated systematic review with novel findings.Crit Rev Oncol Hematol. 2021 Jan;157:103198. doi: 10.1016/j.critrevonc.2020.103198. Epub 2020 Dec 13. Crit Rev Oncol Hematol. 2021. PMID: 33316417
Cited by
-
Cancer treatment and survivorship statistics, 2025.CA Cancer J Clin. 2025 Jul-Aug;75(4):308-340. doi: 10.3322/caac.70011. Epub 2025 May 30. CA Cancer J Clin. 2025. PMID: 40445120 Free PMC article.
-
Cardiovascular Risks and Survival with Abiraterone vs Enzalutamide in Chemotherapy-Naïve Metastatic Castration-Resistant Prostate Cancer in Germany: AVENGER Study.Adv Ther. 2025 Apr;42(4):1919-1934. doi: 10.1007/s12325-025-03132-8. Epub 2025 Mar 2. Adv Ther. 2025. PMID: 40025387 Free PMC article.
References
-
- Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–60. - PubMed
-
- Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20:686–700. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

