A spatiotemporal proteomic map of human adipogenesis
- PMID: 38565923
- PMCID: PMC11132986
- DOI: 10.1038/s42255-024-01025-8
A spatiotemporal proteomic map of human adipogenesis
Abstract
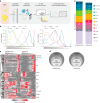
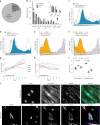
White adipocytes function as major energy reservoirs in humans by storing substantial amounts of triglycerides, and their dysfunction is associated with metabolic disorders; however, the mechanisms underlying cellular specialization during adipogenesis remain unknown. Here, we generate a spatiotemporal proteomic atlas of human adipogenesis, which elucidates cellular remodelling as well as the spatial reorganization of metabolic pathways to optimize cells for lipid accumulation and highlights the coordinated regulation of protein localization and abundance during adipocyte formation. We identify compartment-specific regulation of protein levels and localization changes of metabolic enzymes to reprogramme branched-chain amino acids and one-carbon metabolism to provide building blocks and reduction equivalents. Additionally, we identify C19orf12 as a differentiation-induced adipocyte lipid droplet protein that interacts with the translocase of the outer membrane complex of lipid droplet-associated mitochondria and regulates adipocyte lipid storage by determining the capacity of mitochondria to metabolize fatty acids. Overall, our study provides a comprehensive resource for understanding human adipogenesis and for future discoveries in the field.
© 2024. The Author(s).
Conflict of interest statement
T.D.M. receives research funding from Novo Nordisk and has received speaking fees from Eli Lilly, AstraZeneca and Novo Nordisk. The other authors declare no competing interests.
Figures














References
-
- National Research Council (US) Committee on Diet and Health. Diet and Health: Implications for Reducing Chronic Disease Risk (National Academies Press,1989). - PubMed
MeSH terms
Substances
Grants and funding
- KR5166/2/Deutsche Forschungsgemeinschaft (German Research Foundation)
- TRR 333/1 - 450149205/Deutsche Forschungsgemeinschaft (German Research Foundation)
- TRR296, TRR152, SFB1123 and GRK 2816/1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- NNF20SA0066171/European Foundation for the Study of Diabetes (EFSD)
- NNF22SA0081233/European Foundation for the Study of Diabetes (EFSD)
- NNF20OC0061149/Novo Nordisk Fonden (Novo Nordisk Foundation)
- ERC-CoG Trusted no. 101044445/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- 2023-02839/Vetenskapsrådet (Swedish Research Council)
LinkOut - more resources
Full Text Sources
Research Materials

