B-Myb deficiency boosts bortezomib-induced immunogenic cell death in colorectal cancer
- PMID: 38565963
- PMCID: PMC10987531
- DOI: 10.1038/s41598-024-58424-w
B-Myb deficiency boosts bortezomib-induced immunogenic cell death in colorectal cancer
Abstract
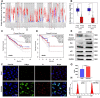
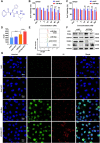
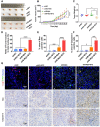
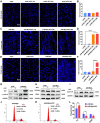
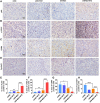
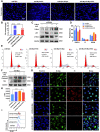
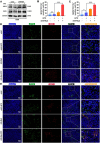
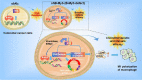
B-Myb has received considerable attention for its critical tumorigenic function of supporting DNA repair. However, its modulatory effects on chemotherapy and immunotherapy have rarely been reported in colorectal cancer. Bortezomib (BTZ) is a novel compound with chemotherapeutic and immunotherapeutic effects, but it fails to work in colorectal cancer with high B-Myb expression. The present study was designed to investigate whether B-Myb deletion in colorectal cancer could potentiate the immune efficacy of BTZ against colorectal cancer and to clarify the underlying mechanism. Stable B-Myb knockdown was induced in colorectal cancer cells, which increased apoptosis of the cancer cells relative to the control group in vitro and in vivo. We found that BTZ exhibited more favourable efficacy in B-Myb-defective colorectal cancer cells and tumor-bearing mice. BTZ treatment led to differential expression of genes enriched in the p53 signaling pathway promoted more powerful downstream DNA damage, and arrested cell cycle in B-Myb-defective colorectal cancer. In contrast, recovery of B-Myb in B-Myb-defective colorectal cancer cells abated BTZ-related DNA damage, cell cycle arrest, and anticancer efficacy. Moreover, BTZ promoted DNA damage-associated enhancement of immunogenicity, as indicated by potentiated expression of HMGB1 and HSP90 in B-Myb-defective cells, thereby driving M1 polarization of macrophages. Collectively, B-Myb deletion in colorectal cancer facilitates the immunogenic death of cancer cells, thereby further promoting the immune efficacy of BTZ by amplifying DNA damage. The present work provides an effective molecular target for colorectal cancer immunotherapy with BTZ.
Keywords: B-Myb (MYBL2); Bioinformatics; Bortezomib (BTZ); DNA damage; Immunogenic death (ICD); Macrophages.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

