Nanoparticles in tumor microenvironment remodeling and cancer immunotherapy
- PMID: 38566199
- PMCID: PMC10986145
- DOI: 10.1186/s13045-024-01535-8
Nanoparticles in tumor microenvironment remodeling and cancer immunotherapy
Abstract
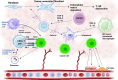
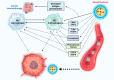
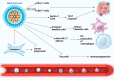
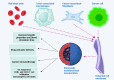
Cancer immunotherapy and vaccine development have significantly improved the fight against cancers. Despite these advancements, challenges remain, particularly in the clinical delivery of immunomodulatory compounds. The tumor microenvironment (TME), comprising macrophages, fibroblasts, and immune cells, plays a crucial role in immune response modulation. Nanoparticles, engineered to reshape the TME, have shown promising results in enhancing immunotherapy by facilitating targeted delivery and immune modulation. These nanoparticles can suppress fibroblast activation, promote M1 macrophage polarization, aid dendritic cell maturation, and encourage T cell infiltration. Biomimetic nanoparticles further enhance immunotherapy by increasing the internalization of immunomodulatory agents in immune cells such as dendritic cells. Moreover, exosomes, whether naturally secreted by cells in the body or bioengineered, have been explored to regulate the TME and immune-related cells to affect cancer immunotherapy. Stimuli-responsive nanocarriers, activated by pH, redox, and light conditions, exhibit the potential to accelerate immunotherapy. The co-application of nanoparticles with immune checkpoint inhibitors is an emerging strategy to boost anti-tumor immunity. With their ability to induce long-term immunity, nanoarchitectures are promising structures in vaccine development. This review underscores the critical role of nanoparticles in overcoming current challenges and driving the advancement of cancer immunotherapy and TME modification.
Keywords: Bioengineered nanostructures; cancer immunotherapy; Immune evasion nanoparticles; Tumor microenvironment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

