MiR26a reverses enzalutamide resistance in a bone-tumor targeted system with an enhanced effect on bone metastatic CRPC
- PMID: 38566211
- PMCID: PMC10985917
- DOI: 10.1186/s12951-024-02438-z
MiR26a reverses enzalutamide resistance in a bone-tumor targeted system with an enhanced effect on bone metastatic CRPC
Abstract
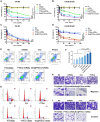
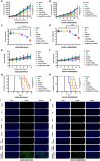
Resistance to androgen receptor (AR) inhibitors, including enzalutamide (Enz), as well as bone metastasis, are major challenges for castration-resistant prostate cancer (CRPC) treatment. In this study, we identified that miR26a can restore Enz sensitivity and inhibit bone metastatic CRPC. To achieve the highest combination effect of miR26a and Enz, we developed a cancer-targeted nano-system (Bm@PT/Enz-miR26a) using bone marrow mesenchymal stem cell (BMSC) membrane and T140 peptide to co-deliver Enz and miR26a. The in vitro/in vivo results demonstrated that miR26a can reverse Enz resistance and synergistically shrink tumor growth, invasion, and metastasis (especially secondary metastasis) in both subcutaneous and bone metastatic CRPC mouse models. We also found that the EZH2/SFRP1/WNT5A axis may be involved in this role. These findings open new avenues for treating bone metastatic and Enz-resistant CRPC.
Keywords: Bone metastatic CRPC; Castration-resistant prostate cancer (CRPC); Drug resistance; Enzalutamide; Nanoparticles.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
The authors declare no competing interests.
Figures





References
-
- Chen WS, Aggarwal R, Zhang L, Zhao SG, Thomas GV, Beer TM, Quigley DA, Foye A, Playdle D, Huang J, Lloyd P, Lu E, Sun D, Guan X, Rettig M, Gleave M, Evans CP, Youngren J, True L, Lara P, Kothari V, Xia Z, Chi KN, Reiter RE, Maher CA, Feng FY, Small EJ, Alumkal JJ. West coast prostate Cancer Dream, genomic drivers of poor prognosis and Enzalutamide Resistance in Metastatic Castration-resistant prostate Cancer. Eur Urol. 2019;76:562–71. doi: 10.1016/j.eururo.2019.03.020. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

