Functional brain networks in Developmental Topographical Disorientation
- PMID: 38566506
- PMCID: PMC10987990
- DOI: 10.1093/cercor/bhae104
Functional brain networks in Developmental Topographical Disorientation
Abstract
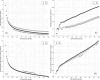
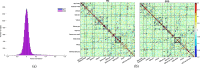
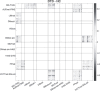
Despite a decade-long study on Developmental Topographical Disorientation, the underlying mechanism behind this neurological condition remains unknown. This lifelong selective inability in orientation, which causes these individuals to get lost even in familiar surroundings, is present in the absence of any other neurological disorder or acquired brain damage. Herein, we report an analysis of the functional brain network of individuals with Developmental Topographical Disorientation ($n = 19$) compared against that of healthy controls ($n = 21$), all of whom underwent resting-state functional magnetic resonance imaging, to identify if and how their underlying functional brain network is altered. While the established resting-state networks (RSNs) are confirmed in both groups, there is, on average, a greater connectivity and connectivity strength, in addition to increased global and local efficiency in the overall functional network of the Developmental Topographical Disorientation group. In particular, there is an enhanced connectivity between some RSNs facilitated through indirect functional paths. We identify a handful of nodes that encode part of these differences. Overall, our findings provide strong evidence that the brain networks of individuals suffering from Developmental Topographical Disorientation are modified by compensatory mechanisms, which might open the door for new diagnostic tools.
Keywords: Developmental Topographical Disorientation; fMRI; functional connectivity; network neuroscience; resting-state networks.
© The Author(s) 2024. Published by Oxford University Press.
Figures




Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Surgical interventions for treating extracapsular hip fractures in older adults: a network meta-analysis.Cochrane Database Syst Rev. 2022 Feb 10;2(2):CD013405. doi: 10.1002/14651858.CD013405.pub2. Cochrane Database Syst Rev. 2022. PMID: 35142366 Free PMC article.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2024 Jan 8;1(1):CD010216. doi: 10.1002/14651858.CD010216.pub8. Cochrane Database Syst Rev. 2024. Update in: Cochrane Database Syst Rev. 2025 Jan 29;1:CD010216. doi: 10.1002/14651858.CD010216.pub9. PMID: 38189560 Free PMC article. Updated.
Cited by
-
Efficacy of functional connectome fingerprinting using tangent-space brain networks.Netw Neurosci. 2025 Apr 30;9(2):549-568. doi: 10.1162/netn_a_00445. eCollection 2025. Netw Neurosci. 2025. PMID: 40487359 Free PMC article.
References
-
- Aguirre GK, D’Esposito M. Topographical disorientation: a synthesis and taxonomy. Brain. 1999:122(9):1613–1628. - PubMed
-
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002:25(12):621–625. - PubMed
-
- Avena-Koenigsberger A, Misic B, Sporns O. Communication dynamics in complex brain networks. Nat Rev Neurosci. 2018:19(1):17–33. - PubMed
-
- Barclay SF, Burles F, Potocki K, Rancourt KM, Nicolson ML, Bech-Hansen NT, Iaria G. Familial aggregation in Developmental Topographical Disorientation (DTD). Cogn Neuropsychol. 2016:33(7–8):388–397. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

