Neurodevelopmental defects in a mouse model of O-GlcNAc transferase intellectual disability
- PMID: 38566589
- PMCID: PMC11095632
- DOI: 10.1242/dmm.050671
Neurodevelopmental defects in a mouse model of O-GlcNAc transferase intellectual disability
Abstract
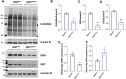
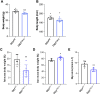
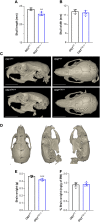
The addition of O-linked β-N-acetylglucosamine (O-GlcNAc) to proteins (referred to as O-GlcNAcylation) is a modification that is crucial for vertebrate development. O-GlcNAcylation is catalyzed by O-GlcNAc transferase (OGT) and reversed by O-GlcNAcase (OGA). Missense variants of OGT have recently been shown to segregate with an X-linked syndromic form of intellectual disability, OGT-linked congenital disorder of glycosylation (OGT-CDG). Although the existence of OGT-CDG suggests that O-GlcNAcylation is crucial for neurodevelopment and/or cognitive function, the underlying pathophysiologic mechanisms remain unknown. Here we report a mouse line that carries a catalytically impaired OGT-CDG variant. These mice show altered O-GlcNAc homeostasis with decreased global O-GlcNAcylation and reduced levels of OGT and OGA in the brain. Phenotypic characterization of the mice revealed lower body weight associated with reduced body fat mass, short stature and microcephaly. This mouse model will serve as an important tool to study genotype-phenotype correlations in OGT-CDG in vivo and for the development of possible treatment avenues for this disorder.
Keywords: O-GlcNAcylation; Intellectual disability; Vertebrate development.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

