Short versus long-acting erythropoiesis-stimulating agents for anemia management in Egyptian hemodialysis patients
- PMID: 38567102
- PMCID: PMC10985414
- DOI: 10.5339/qmj.2024.16
Short versus long-acting erythropoiesis-stimulating agents for anemia management in Egyptian hemodialysis patients
Abstract
Background: Chronic kidney disease (CKD) often results in renal anemia, impacting the well-being of patients and causing various negative consequences. Erythropoiesis-stimulating agents (ESAs) offer promising solutions for managing anemia in CKD. This study aimed to evaluate and compare the effectiveness, safety profile, and cost-effectiveness of short-acting (Eprex®) and long-acting (Aranesp®) ESAs.
Method: This comparative prospective cohort cost-effectiveness study was carried out over 6 months among adult Egyptian hemodialysis patients of either gender. Participants were categorized into two groups based on the type of ESA administered: the Eprex group, receiving epoetin alfa, and the Aranesp group, receiving darbepoetin alfa. These two treatment groups' efficacy, safety, and cost were analyzed and compared.
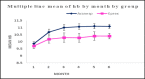
Results: Of 127 hemodialysis patients, 60 (47.2%) received Eprex, while 67 (52.8%) were treated with Aranesp. Target hemoglobin (Hb) was achieved by 50.6% of patients in the Eprex group versus 63.4% in the Aranesp group, with a significant difference (P < 0.001). Both treatment groups exhibited a similar safety profile, while Aranesp® was considered the cost-saving protocol.
Conclusion: In hemodialysis Egyptian patients, Aranesp with extended dosing intervals proved to be more effective in achieving target Hb with comparable adverse effect profiles, a substantial cost-saving strategy, and offered time-saving advantages for medical staff workload compared to Eprex.
Trial registration: The Clinicaltrial.gov registration ID is NCT05699109 (26/01/2023).
Keywords: Short-/long-acting ESAs; anemia; chronic renal failure; hemodialysis; pharmacoeconomics.
© 2024 Soliman, Magdy, Ayoub, Ebid, Licensee HBKU Press.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Comparison between short- and long-acting erythropoiesis-stimulating agents in hemodialysis patients: target hemoglobin, variability, and outcome.Int Urol Nephrol. 2014 Feb;46(2):453-9. doi: 10.1007/s11255-013-0640-7. Epub 2014 Jan 22. Int Urol Nephrol. 2014. PMID: 24448756 Free PMC article. Clinical Trial.
-
Darbepoetin alfa injection versus epoetin alfa injection for treating anemia of Chinese hemodialysis patients with chronic kidney failure: A randomized, open-label, parallel-group, non-inferiority Phase III trail.Chronic Dis Transl Med. 2022 Mar 29;8(1):59-70. doi: 10.1002/cdt3.13. eCollection 2022 Mar. Chronic Dis Transl Med. 2022. PMID: 35620165 Free PMC article.
-
Incidence of erythropoietin antibody-mediated pure red cell aplasia: the Prospective Immunogenicity Surveillance Registry (PRIMS).Nephrol Dial Transplant. 2015 Mar;30(3):451-60. doi: 10.1093/ndt/gfu297. Epub 2014 Sep 19. Nephrol Dial Transplant. 2015. PMID: 25239637 Free PMC article.
-
Differentiating factors between erythropoiesis-stimulating agents: an update to selection for anaemia of chronic kidney disease.Drugs. 2013 Feb;73(2):117-30. doi: 10.1007/s40265-012-0002-2. Drugs. 2013. PMID: 23338536 Review.
-
[Treating anaemia in patients with chronic kidney disease: what evidence for using ESAs, after a 30-year journey?].G Ital Nefrol. 2020 Aug 11;37(4):2020-vol4. G Ital Nefrol. 2020. PMID: 32809282 Review. Italian.
References
-
- Hassaballa M, El-Wakil H, Elsharkawy M, Khamis S, El Tantawy T, Wahby W, et al. Egyptian renal data system (ERDS) 2020: an annual report of end-stage kidney disease patients on regular hemodialysis. J Egypt Soc Nephrol Transplant. 2022;;22((1):):1–28. doi: 10.4103/jesnt.jesnt_37_21. doi: - DOI
-
- Regidor DL, Kopple JD, Kovesdy CP, Kilpatrick RD, McAllister CJ, Aronovitz J, et al. Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients . J Am Soc Nephrol. 2006;;17((4):):1181–91. doi: 10.1681/ASN.2005090997. doi: - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical