Is the leptin/BMI ratio a reliable biomarker for endometriosis?
- PMID: 38567305
- PMCID: PMC10985179
- DOI: 10.3389/fendo.2024.1359182
Is the leptin/BMI ratio a reliable biomarker for endometriosis?
Abstract
Background: The aim of this study was to analyze the concentration of leptin in peritoneal fluid and plasma and to assess their role as potential biomarkers in the diagnosis of endometriosis.
Materials & methods: Leptin adjusted for BMI (leptin/BMI ratio) was measured using surface plasmon resonance imaging (SPRI) biosensors. Patients with suspected endometriosis were included in the study. Plasma was collected from 70 cases, and peritoneal fluid from 67 cases. Based on the presence of endometriosis lesions detected during laparoscopy, patients were divided into a study group and a control group (patients without endometriosis).
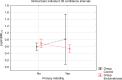
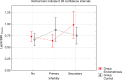
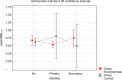
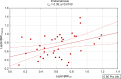
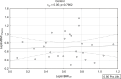
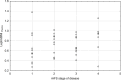
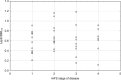
Results: Leptin/BMI ratio in plasma did not differ between women with endometriosis and the control group (0.7159 ± 0.259 vs 0.6992 ± 0.273, p= 0,7988). No significant differences were observed in peritoneal leptin/BMI ratio levels in patients with and without endometriosis (0.6206 ± 0.258 vs 0.6215 ± 0.264, p= 0,9896). Plasma and peritoneal leptin/BMI ratios were significantly lower in women with endometriosis - related primary infertility compared to women with endometriosis without primary infertility (0.640 ± 0.502 vs 0.878 ± 0.623, p < 0.05). The difference was observed in case of primary infertility, but not in terms of the secondary one. No significant differences were noted between leptin/BMI ratio in the proliferative phase and the secretory phase (0.716 ± 0.252 vs 0.697 ± 0.288, p= 0,7785).
Conclusion: The results of present study do not support the relevance of leptin concentration determination as a biomarker of the endometriosis. Due to the limited number of samples in the tested group, further studies are needed to confirm its role.
Keywords: endometriosis; infertility; leptin; peritoneal fluid; plasma.
Copyright © 2024 Zyguła, Sankiewicz, Sakowicz, Dobrzyńska, Dakowicz, Mańka, Kiecka, Spaczynski, Piekarski, Banaszewska, Jakimiuk, Issat, Rokita, Młodawski, Szubert, Sieroszewski, Raba, Szczupak, Kluza, Kluza, Pierzyński, Wojtyla, Lipa, Warzecha, Wielgos, Cendrowski, Gorodkiewicz and Laudanski.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

