Real-world persistence of multiple sclerosis disease-modifying therapies
- PMID: 38567516
- PMCID: PMC11235620
- DOI: 10.1111/ene.16289
Real-world persistence of multiple sclerosis disease-modifying therapies
Abstract
Background and purpose: Treatment persistence is the continuation of therapy over time. It reflects a combination of treatment efficacy and tolerability. We aimed to describe real-world rates of persistence on disease-modifying therapies (DMTs) for people with multiple sclerosis (pwMS) and reasons for DMT discontinuation.
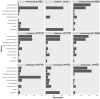
Methods: Treatment data on 4366 consecutive people with relapse-onset multiple sclerosis (MS) were pooled from 13 UK specialist centres during 2021. Inclusion criteria were exposure to at least one MS DMT and a complete history of DMT prescribing. PwMS in blinded clinical trials were excluded. Data collected included sex, age at MS onset, age at DMT initiation, DMT treatment dates, and reasons for stopping or switching DMT. For pwMS who had received immune reconstituting therapies (cladribine/alemtuzumab), discontinuation date was defined as starting an alternative DMT. Kaplan-Meier survival analyses were used to express DMT persistence.
Results: In 6997 treatment events (1.6 per person with MS), median time spent on any single maintenance DMT was 4.3 years (95% confidence interval = 4.1-4.5 years). The commonest overall reasons for DMT discontinuation were adverse events (35.0%) and lack of efficacy (30.3%). After 10 years, 20% of people treated with alemtuzumab had received another subsequent DMT, compared to 82% of people treated with interferon or glatiramer acetate.
Conclusions: Immune reconstituting DMTs may have the highest potential to offer a single treatment for relapsing MS. Comparative data on DMT persistence and reasons for discontinuation are valuable to inform treatment decisions and in personalizing treatment in MS.
Keywords: disease‐modifying therapy; multiple sclerosis; persistence; treatment.
© 2024 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
C.M.R. has received research funding from Sanofi. E.C.T. has received honoraria for consulting work, or speaker fees, from Biogen, Janssen, Merck, Novartis, and Roche, and has received travel grants to attend or speak at educational meetings from Biogen, Merck, Roche, and Novartis. G.I. has received honoraria for consulting work, or speaker fees, from Biogen, Novartis, and Roche, and has received travel grants to attend or speak at educational meetings from Biogen, Merck, Roche, and Novartis. H.L.F. has received honoraria for consulting work and/or educational activities from Biogen, Merck, Novartis, Roche, Sanofi Genzyme, and Teva. I.G. has received a research grant from Merck‐Serono. J.T.M.M. has received honoraria for consulting work, or speaker fees, from Biogen, Celegene BMS, Janssen, Merck, Novartis, Roche, Sandoz, and Sanofi. She has received travel grants to attend or speak at educational meetings from Biogen, Janssen, Merck, Novartis, Roche, Sandoz, and Sanofi. K.E.H. has received honoraria for consulting work, or speaker fees, from Biogen, Merck, and Roche, and has received travel grants to attend or speak at educational meetings from Biogen, Merck, Roche, and Novartis. M.J.R. has received speaker fees from Biogen. O.M.G. has received honoraria as a consultant on scientific advisory boards for Genzyme, Biogen, Merck, Roche, and Novartis; has received travel grants from Biogen, Merck, Roche, Sanofi, and Novartis; has participated in clinical trials by Biogen; and has received research funding from Biogen. R.D. has received educational/personal honoraria (to her organization) from Roche, Novartis, Sandoz, Biogen, and Merck, and research funding from Biogen and Merck. S.E.H. has received honoraria for consulting work, or speaker fees, from Biogen, Merck, Novartis, and Roche. She has received travel grants to attend conferences from Biogen, Merck, Roche, and Novartis. S.K. has received honoraria for consulting work from Biogen and Novartis. She has received travel grants to attend or speak at educational meetings from Biogen and Novartis. T.A. has received honoraria for advisory work, speaker fees, or research grants from Biogen, Merck, Novartis, Roche, and Janssen. T.E.W. has received honoraria for educational talks from Novartis and Merck. None of the other authors has any conflict of interest to disclose.
Figures



References
-
- Trojano M, Tintore M, Montalban X, et al. Treatment decisions in multiple sclerosis – insights from real‐world observational studies. Nat Rev Neurol. 2017;13(2):105‐118. - PubMed
-
- Hua LH, Harris H, Conway D, Hersh CM. Disease activity outcomes with different washout periods after switching from natalizumab to an alternative disease‐modifying therapy. J Neurol. 2020;267(8):2214‐2220. - PubMed
-
- Manzano A, Eskyte I, Ford HL, et al. Patient perspective on decisions to switch disease‐modifying treatments in relapsing‐remitting multiple sclerosis. Mult Scler Relat Disord. 2020;46:102507. - PubMed
-
- Kozlicki MZ, Markley B, Shah NB, DeClercq J, Choi L, Zuckerman AD. A cross‐sectional analysis of persistence to disease‐modifying therapies in treatment naive and experienced patients with relapsing multiple sclerosis at a health‐system specialty pharmacy. Mult Scler Relat Disord. 2022;63:103860. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

