Peptoid-Cross-Linked Hydrogel Stiffness Modulates Human Mesenchymal Stromal Cell Immunoregulatory Potential in the Presence of Interferon-Gamma
- PMID: 38567626
- PMCID: PMC11250919
- DOI: 10.1002/mabi.202400111
Peptoid-Cross-Linked Hydrogel Stiffness Modulates Human Mesenchymal Stromal Cell Immunoregulatory Potential in the Presence of Interferon-Gamma
Abstract
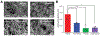
Human mesenchymal stromal cell (hMSC) manufacturing requires the production of large numbers of therapeutically potent cells. Licensing with soluble cytokines improves hMSC therapeutic potency by enhancing secretion of immunoactive factors but typically decreases proliferative ability. Soft hydrogels, however, have shown promise for boosting immunomodulatory potential, which may compensate for decreased proliferation. Here, hydrogels are cross-linked with peptoids of different secondary structures to generate substrates of various bulk stiffnesses but fixed network connectivity. Secretions of interleukin 6, monocyte chemoattractive protein-1, macrophage colony-stimulating factor, and vascular endothelial growth factor are shown to depend on hydrogel stiffness in the presence of interferon gamma (IFN-γ) supplementation, with soft substrates further improving secretion. The immunological function of these secreted cytokines is then investigated via coculture of hMSCs seeded on hydrogels with primary peripheral blood mononuclear cells (PBMCs) in the presence and absence of IFN-γ. Cocultures with hMSCs seeded on softer hydrogels show decreased PBMC proliferation with IFN-γ. To probe possible signaling pathways, immunofluorescent studies probe the nuclear factor kappa B pathway and demonstrate that IFN-γ supplementation and softer hydrogel mechanics lead to higher activation of this pathway. Overall, these studies may allow for production of more efficacious therapeutic hMSCs in the presence of IFN-γ.
Keywords: human mesenchymal stromal cells; hydrogels; immunosuppression; secretome.
© 2024 Wiley‐VCH GmbH.
Conflict of interest statement
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





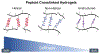
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

