Integrative analysis of DNA replication origins and ORC-/MCM-binding sites in human cells reveals a lack of overlap
- PMID: 38567819
- PMCID: PMC10990492
- DOI: 10.7554/eLife.89548
Integrative analysis of DNA replication origins and ORC-/MCM-binding sites in human cells reveals a lack of overlap
Abstract
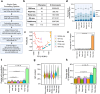
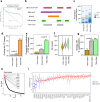
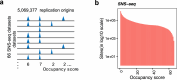
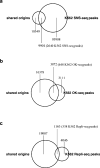
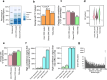
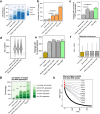
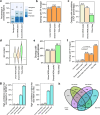
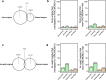
Based on experimentally determined average inter-origin distances of ~100 kb, DNA replication initiates from ~50,000 origins on human chromosomes in each cell cycle. The origins are believed to be specified by binding of factors like the origin recognition complex (ORC) or CTCF or other features like G-quadruplexes. We have performed an integrative analysis of 113 genome-wide human origin profiles (from five different techniques) and five ORC-binding profiles to critically evaluate whether the most reproducible origins are specified by these features. Out of ~7.5 million union origins identified by all datasets, only 0.27% (20,250 shared origins) were reproducibly obtained in at least 20 independent SNS-seq datasets and contained in initiation zones identified by each of three other techniques, suggesting extensive variability in origin usage and identification. Also, 21% of the shared origins overlap with transcriptional promoters, posing a conundrum. Although the shared origins overlap more than union origins with constitutive CTCF-binding sites, G-quadruplex sites, and activating histone marks, these overlaps are comparable or less than that of known transcription start sites, so that these features could be enriched in origins because of the overlap of origins with epigenetically open, promoter-like sequences. Only 6.4% of the 20,250 shared origins were within 1 kb from any of the ~13,000 reproducible ORC-binding sites in human cancer cells, and only 4.5% were within 1 kb of the ~11,000 union MCM2-7-binding sites in contrast to the nearly 100% overlap in the two comparisons in the yeast, Saccharomyces cerevisiae. Thus, in human cancer cell lines, replication origins appear to be specified by highly variable stochastic events dependent on the high epigenetic accessibility around promoters, without extensive overlap between the most reproducible origins and currently known ORC- or MCM-binding sites.
Keywords: DNA replication; MCM2-7; ORC; chromosomes; gene expression; human; integrative analysis; origins of replication.
© 2023, Tian et al.
Conflict of interest statement
MT, ZW, ZS, ES, YS, AD, CZ No competing interests declared
Figures





Update of
-
Integrative analysis of DNA replication origins and ORC/MCM binding sites in human cells reveals a lack of overlap.bioRxiv [Preprint]. 2023 Nov 30:2023.07.25.550556. doi: 10.1101/2023.07.25.550556. bioRxiv. 2023. Update in: Elife. 2024 Apr 03;12:RP89548. doi: 10.7554/eLife.89548. PMID: 37546918 Free PMC article. Updated. Preprint.
References
-
- Akerman I, Kasaai B, Bazarova A, Sang PB, Peiffer I, Artufel M, Derelle R, Smith G, Rodriguez-Martinez M, Romano M, Kinet S, Tino P, Theillet C, Taylor N, Ballester B, Méchali M. A predictable conserved DNA base composition signature defines human core DNA replication origins. Nature Communications. 2020;11:4826. doi: 10.1038/s41467-020-18527-0. - DOI - PMC - PubMed
-
- Andrews S. FastQC: A quality control tool for high throughput sequence data. 0.11.9Babraham Bioinformatics. 2010 https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
-
- Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, Yefanov A, Lee H, Zhang N, Robertson CL, Serova N, Davis S, Soboleva A. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Research. 2013;41:D991–D995. doi: 10.1093/nar/gks1193. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

