Development, synthesis and validation of improved c-Myc/Max inhibitors
- PMID: 38568057
- PMCID: PMC10989597
- DOI: 10.1111/jcmm.18272
Development, synthesis and validation of improved c-Myc/Max inhibitors
Abstract
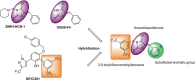
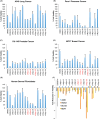
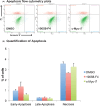
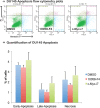
The pathophysiological foundations of various diseases are often subject to alteration through the utilization of small compounds, rendering them invaluable tools for the exploration and advancement of novel therapeutic strategies. Within the scope of this study, we meticulously curated a diverse library of novel small compounds meticulously designed to specifically target the c-Myc/Max complex. We conducted in vitro examinations of novel c-Myc inhibitors across a spectrum of cancer cell lines, including PANC1 (pancreatic adenocarcinoma), MCF7 (breast carcinoma), DU-145 (prostate carcinoma), and A549 (lung cancer). The initial analysis involved a 25 μM dose, which enabled the identification of potent anticancer compounds effective against a variety of tumour types. We identified c-Myc inhibitors with remarkable potency, featuring IC50 values as low as 1.6 μM and up to 40 times more effective than the reference molecule in diminishing cancer cell viability. Notably, c-Myc-i7 exhibited exceptional selectivity, displaying 37-fold and 59-fold preference for targeting prostate and breast cancers, respectively, over healthy cells. Additionally, we constructed drug-likeness models. This study underscores the potential for in vitro investigations of various tumour types using novel c-Myc inhibitors to yield ground-breaking and efficacious anticancer compounds.
Keywords: Max; anti‐cancer; c‐Myc; rhodanine; ultrasonication.
© 2024 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest to disclose.
Figures







References
-
- Unsoy G, Gunduz U. Smart drug delivery systems in cancer therapy. Curr Drug Targets. 2018;19(3):202‐212. - PubMed
-
- Erol A. Deciphering the intricate regulatory mechanisms for the cellular choice between cell repair, apoptosis or senescence in response to damaging signals. Cell Signal. 2011;23(7):1076‐1081. - PubMed
-
- Ashrafizadeh M, Zarabi A, Hushmandi K, et al. c‐Myc signaling pathway in treatment and prevention of brain tumors. Curr Cancer Drug Targets. 2021;21(1):2‐20. - PubMed
-
- Faskhoudi MA, Molaei P, Sadrkhanloo M, et al. Molecular landscape of c‐Myc signaling in prostate cancer: a roadmap to clinical translation. Pathol Res Pract. 2022;233:153851. - PubMed
-
- Aksoz M, Albayrak E, Aslan GS, et al. c‐Myc inhibitor 10074‐G5 induces murine and human hematopoietic stem and progenitor cell expansion and HDR modulator Rad51 expression. Curr Cancer Drug Targets. 2019;19(6):479‐494. - PubMed
List of Relevant Articles
-
- Mermer A, Bulbul MV, Kalender SM, Keskin I, Tuzun B, Eyupoglu OE. Benzotriazole‐oxadiazole hybrid compounds: synthesis, anticancer activity, molecular docking and ADME profiling studies. J Mol Liq. 2022;359:119264. doi:10.1016/j.molliq.2022.119264 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

