pH-dependent regulation of an acidophilic O-acetylhomoserine sulfhydrylase from Lactobacillus plantarum
- PMID: 38568076
- PMCID: PMC11107162
- DOI: 10.1128/aem.00118-24
pH-dependent regulation of an acidophilic O-acetylhomoserine sulfhydrylase from Lactobacillus plantarum
Abstract
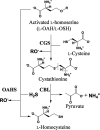
Bacteria have two routes for the l-methionine biosynthesis. In one route called the direct sulfuration pathway, acetylated l-homoserine is directly converted into l-homocysteine. The reaction using H2S as the second substrate is catalyzed by a pyridoxal 5'-phosphate-dependent enzyme, O-acetylhomoserine sulfhydrylase (OAHS). In the present study, we determined the enzymatic functions and the structures of OAHS from Lactobacillus plantarum (LpOAHS). The LpOAHS enzyme exhibited the highest catalytic activity under the weak acidic pH condition. In addition, crystallographic analysis revealed that the enzyme takes two distinct structures, open and closed forms. In the closed form, two acidic residues are sterically clustered. The proximity may cause the electrostatic repulsion, inhibiting the formation of the closed form under the neutral to the basic pH conditions. We concluded that the pH-dependent regulation mechanism using the two acidic residues contributes to the acidophilic feature of the enzyme.
Importance: In the present study, we can elucidate the pH-dependent regulation mechanism of the acidophilic OAHS. The acidophilic feature of the enzyme is caused by the introduction of an acidic residue to the neighborhood of the key acidic residue acting as a switch for the structural interconversion. The strategy may be useful in the field of protein engineering to change the optimal pH of the enzymes. In addition, this study may be useful for the development of antibacterial drugs because the l-methionine synthesis essential for bacteria is inhibited by the OAHS inhibitors. The compounds that can inhibit the interconversion between the open and closed forms of OAHS may become antibacterial drugs.
Keywords: Lactobacillus plantarum; O-acetylhomoserine sulfhydrylase; acidophilicity; l-methionine; pH-dependent regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Identification of O-acetylhomoserine sulfhydrylase, a putative enzyme responsible for methionine biosynthesis in Clostridioides difficile: Gene cloning and biochemical characterizations.IUBMB Life. 2019 Nov;71(11):1815-1823. doi: 10.1002/iub.2139. Epub 2019 Jul 30. IUBMB Life. 2019. PMID: 31359602
-
Methionine biosynthesis in Brevibacterium flavum: properties and essential role of O-acetylhomoserine sulfhydrylase.J Biochem. 1982 Apr;91(4):1163-71. doi: 10.1093/oxfordjournals.jbchem.a133799. J Biochem. 1982. PMID: 7096282
-
O-acetylhomoserine sulfhydrylase from Clostridium novyi. Cloning, expression of the gene and characterization of the enzyme.Protein Expr Purif. 2021 Apr;180:105810. doi: 10.1016/j.pep.2020.105810. Epub 2020 Dec 16. Protein Expr Purif. 2021. PMID: 33338587
-
A novel mechanism of sulfur transfer catalyzed by O-acetylhomoserine sulfhydrylase in the methionine-biosynthetic pathway of Wolinella succinogenes.Acta Crystallogr D Biol Crystallogr. 2011 Oct;67(Pt 10):831-8. doi: 10.1107/S0907444911028010. Epub 2011 Sep 8. Acta Crystallogr D Biol Crystallogr. 2011. PMID: 21931214 Free PMC article.
-
Roles of O-acetyl-L-homoserine sulfhydrylases in micro-organisms.Biochimie. 1989 Nov-Dec;71(11-12):1125-43. doi: 10.1016/0300-9084(89)90016-3. Biochimie. 1989. PMID: 2517474 Review.
Cited by
-
Microbiome-mediated colonization resistance to carbapenem-resistant Klebsiella pneumoniae in ICU patients.NPJ Biofilms Microbiomes. 2025 Aug 9;11(1):157. doi: 10.1038/s41522-025-00791-x. NPJ Biofilms Microbiomes. 2025. PMID: 40783567 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

