Trends in SARS-CoV-2 seroprevalence among pregnant women attending first antenatal care visits in Zambia: A repeated cross-sectional survey, 2021-2022
- PMID: 38568905
- PMCID: PMC10990173
- DOI: 10.1371/journal.pgph.0003073
Trends in SARS-CoV-2 seroprevalence among pregnant women attending first antenatal care visits in Zambia: A repeated cross-sectional survey, 2021-2022
Abstract
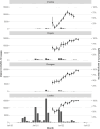
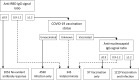
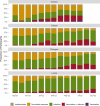
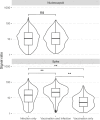
SARS-CoV-2 serosurveys help estimate the extent of transmission and guide the allocation of COVID-19 vaccines. We measured SARS-CoV-2 seroprevalence among women attending ANC clinics to assess exposure trends over time in Zambia. We conducted repeated cross-sectional SARS-CoV-2 seroprevalence surveys among pregnant women aged 15-49 years attending their first ANC visits in four districts of Zambia (two urban and two rural) during September 2021-September 2022. Serologic testing was done using a multiplex bead assay which detects IgG antibodies to the nucleocapsid protein and the spike protein receptor-binding domain (RBD). We calculated monthly SARS-CoV-2 seroprevalence by district. We also categorized seropositive results as infection alone, infection and vaccination, or vaccination alone based on anti-RBD and anti-nucleocapsid test results and self-reported COVID-19 vaccination status (vaccinated was having received ≥1 dose). Among 8,304 participants, 5,296 (63.8%) were cumulatively seropositive for SARS-CoV-2 antibodies from September 2021 through September 2022. SARS-CoV-2 seroprevalence primarily increased from September 2021 to September 2022 in three districts (Lusaka: 61.8-100.0%, Chongwe: 39.6-94.7%, Chipata: 56.5-95.0%), but in Chadiza, seroprevalence increased from 27.8% in September 2021 to 77.2% in April 2022 before gradually dropping to 56.6% in July 2022. Among 5,906 participants with a valid COVID-19 vaccination status, infection alone accounted for antibody responses in 77.7% (4,590) of participants. Most women attending ANC had evidence of prior SARS-CoV-2 infection and most SARS-CoV-2 seropositivity was infection-induced. Capturing COVID-19 vaccination status and using a multiplex bead assay with anti-nucleocapsid and anti-RBD targets facilitated distinguishing infection-induced versus vaccine-induced antibody responses during a period of increasing COVID-19 vaccine coverage in Zambia. Declining seroprevalence in Chadiza may indicate waning antibodies and a need for booster vaccines. ANC clinics have a potential role in ongoing SARS-CoV-2 serosurveillance and can continue to provide insights into SARS-CoV-2 antibody dynamics to inform near real-time public health responses.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Implementing SARS-CoV-2 routine surveillance in antenatal care in Zambia, 2021-2022: best practices and lessons learned.BMC Public Health. 2025 Feb 28;25(1):813. doi: 10.1186/s12889-025-21918-x. BMC Public Health. 2025. PMID: 40021963 Free PMC article.
-
Evaluating immunity to SARS-CoV-2 in nursing home residents using saliva IgG.J Am Geriatr Soc. 2022 Mar;70(3):659-668. doi: 10.1111/jgs.17660. Epub 2022 Jan 22. J Am Geriatr Soc. 2022. PMID: 35038344
-
Seroprevalence of SARS-CoV-2-specific anti-spike IgM, IgG, and anti-nucleocapsid IgG antibodies during the second wave of the pandemic: A population-based cross-sectional survey across Kashmir, India.Front Public Health. 2022 Oct 6;10:967447. doi: 10.3389/fpubh.2022.967447. eCollection 2022. Front Public Health. 2022. PMID: 36276377 Free PMC article.
-
Anti-SARS-CoV-2 IgG Seroprevalence in Tyrol, Austria, among 28,768 Blood Donors between May 2022 and March 2023.Vaccines (Basel). 2024 Mar 8;12(3):284. doi: 10.3390/vaccines12030284. Vaccines (Basel). 2024. PMID: 38543918 Free PMC article.
-
Systematic literature review of SARS-CoV-2 seroprevalence surveys in Canada through April 2021.IJID Reg. 2022 Sep;4:157-164. doi: 10.1016/j.ijregi.2022.07.010. Epub 2022 Jul 29. IJID Reg. 2022. PMID: 35919829 Free PMC article. Review.
Cited by
-
Implementing SARS-CoV-2 routine surveillance in antenatal care in Zambia, 2021-2022: best practices and lessons learned.BMC Public Health. 2025 Feb 28;25(1):813. doi: 10.1186/s12889-025-21918-x. BMC Public Health. 2025. PMID: 40021963 Free PMC article.
-
COVID-19 vaccine uptake and associated risk factors among first antenatal care attendees in Zambia, 2021-2022: A repeated cross-sectional study.PLOS Glob Public Health. 2024 Oct 24;4(10):e0003028. doi: 10.1371/journal.pgph.0003028. eCollection 2024. PLOS Glob Public Health. 2024. PMID: 39446709 Free PMC article.
References
-
- Zambia National Public Health Institute. Zambia COVID-19 Dashboard. Mar 14, 2023. https://www.arcgis.com/apps/dashboards/3b3a01c1d8444932ba075fb44b119b63 (accessed Apr 13, 2023).
-
- National Institute for Communicable Diseases. SARS-CoV-2 seroprevalence in the Cape Town Metropolitan Subdistricts after the peak of infections. COVID-19-Special-Public-Health-Surveillance-Bulletin_Issue-5.pdf (accessed May 15, 2023).
LinkOut - more resources
Full Text Sources
Miscellaneous
