Postpandemic Sentinel Surveillance of Respiratory Diseases in the Context of the World Health Organization Mosaic Framework: Protocol for a Development and Evaluation Study Involving the English Primary Care Network 2023-2024
- PMID: 38569175
- PMCID: PMC11024753
- DOI: 10.2196/52047
Postpandemic Sentinel Surveillance of Respiratory Diseases in the Context of the World Health Organization Mosaic Framework: Protocol for a Development and Evaluation Study Involving the English Primary Care Network 2023-2024
Abstract
Background: Prepandemic sentinel surveillance focused on improved management of winter pressures, with influenza-like illness (ILI) being the key clinical indicator. The World Health Organization (WHO) global standards for influenza surveillance include monitoring acute respiratory infection (ARI) and ILI. The WHO's mosaic framework recommends that the surveillance strategies of countries include the virological monitoring of respiratory viruses with pandemic potential such as influenza. The Oxford-Royal College of General Practitioner Research and Surveillance Centre (RSC) in collaboration with the UK Health Security Agency (UKHSA) has provided sentinel surveillance since 1967, including virology since 1993.
Objective: We aim to describe the RSC's plans for sentinel surveillance in the 2023-2024 season and evaluate these plans against the WHO mosaic framework.
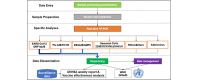
Methods: Our approach, which includes patient and public involvement, contributes to surveillance objectives across all 3 domains of the mosaic framework. We will generate an ARI phenotype to enable reporting of this indicator in addition to ILI. These data will support UKHSA's sentinel surveillance, including vaccine effectiveness and burden of disease studies. The panel of virology tests analyzed in UKHSA's reference laboratory will remain unchanged, with additional plans for point-of-care testing, pneumococcus testing, and asymptomatic screening. Our sampling framework for serological surveillance will provide greater representativeness and more samples from younger people. We will create a biomedical resource that enables linkage between clinical data held in the RSC and virology data, including sequencing data, held by the UKHSA. We describe the governance framework for the RSC.
Results: We are co-designing our communication about data sharing and sampling, contextualized by the mosaic framework, with national and general practice patient and public involvement groups. We present our ARI digital phenotype and the key data RSC network members are requested to include in computerized medical records. We will share data with the UKHSA to report vaccine effectiveness for COVID-19 and influenza, assess the disease burden of respiratory syncytial virus, and perform syndromic surveillance. Virological surveillance will include COVID-19, influenza, respiratory syncytial virus, and other common respiratory viruses. We plan to pilot point-of-care testing for group A streptococcus, urine tests for pneumococcus, and asymptomatic testing. We will integrate test requests and results with the laboratory-computerized medical record system. A biomedical resource will enable research linking clinical data to virology data. The legal basis for the RSC's pseudonymized data extract is The Health Service (Control of Patient Information) Regulations 2002, and all nonsurveillance uses require research ethics approval.
Conclusions: The RSC extended its surveillance activities to meet more but not all of the mosaic framework's objectives. We have introduced an ARI indicator. We seek to expand our surveillance scope and could do more around transmissibility and the benefits and risks of nonvaccine therapies.
Keywords: COVID-19; World Health Organization; computerized medical record system; human influenza; influenza vaccines; pandemic; phenotype; respiratory syncytial virus; respiratory tract infections; sentinel surveillance; vaccination.
©Xinchun Gu, Conall Watson, Utkarsh Agrawal, Heather Whitaker, William H. Elson, Sneha Anand, Ray Borrow, Anna Buckingham, Elizabeth Button, Lottie Curtis, Dominic Dunn, Alex J. Elliot, Filipa Ferreira, Rosalind Goudie, Uy Hoang, Katja Hoschler, Gavin Jamie, Debasish Kar, Beatrix Kele, Meredith Leston, Ezra Linley, Jack Macartney, Gemma L Marsden, Cecilia Okusi, Omid Parvizi, Catherine Quinot, Praveen Sebastianpillai, Vanashree Sexton, Gillian Smith, Timea Suli, Nicholas P B Thomas, Catherine Thompson, Daniel Todkill, Rashmi Wimalaratna, Matthew Inada-Kim, Nick Andrews, Victoria Tzortziou-Brown, Rachel Byford, Maria Zambon, Jamie Lopez-Bernal, Simon de Lusignan. Originally published in JMIR Public Health and Surveillance (https://publichealth.jmir.org), 03.04.2024.
Conflict of interest statement
Conflicts of Interest: SdL has received funding through his university from Astra-Zeneca, Eli-Lilly, GSK, MSD, Novo Nordisk, Sanofi, Seqirus, and Takeda, and has been a member of advisory boards for Astra- Zeneca, Sanofi, and Seqirus. He is the Director of the Oxford-Royal College of General Practitioner Research and Surveillance Centre. MZ is the chair of the charitable organization International Society for Influenza and other Respiratory Viruses (ISIRV) (not remunerated) and a member of the UK Government Scientific Advisory Groups Scientific Advisory Group for Emergencies (SAGE), New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG), and Joint Committee on Vaccination and Immunization (JCVI) (not remunerated). UH has received funding from Sanofi for vaccine-related workshops and has been a member of the advisory board for Janssen. All other authors declare no conflicts of interest.
Figures










References
-
- Bitterman R, Eliakim-Raz N, Vinograd I, Zalmanovici Trestioreanu A, Leibovici L, Paul M. Influenza vaccines in immunosuppressed adults with cancer. Cochrane Database Syst Rev. 2018 Feb 01;2(2):CD008983. doi: 10.1002/14651858.CD008983.pub3. https://europepmc.org/abstract/MED/29388675 - DOI - PMC - PubMed
-
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2018 Feb 01;2(2):CD001269. doi: 10.1002/14651858.CD001269.pub6. https://europepmc.org/abstract/MED/29388196 - DOI - PMC - PubMed
-
- Jefferson T, Rivetti A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2018 Feb 01;2(2):CD004879. doi: 10.1002/14651858.CD004879.pub5. https://europepmc.org/abstract/MED/29388195 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

