Three-dimensional imaging studies in mice identify cellular dynamics of skeletal muscle regeneration
- PMID: 38569550
- PMCID: PMC11153043
- DOI: 10.1016/j.devcel.2024.03.017
Three-dimensional imaging studies in mice identify cellular dynamics of skeletal muscle regeneration
Abstract
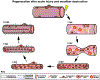
The function of many organs, including skeletal muscle, depends on their three-dimensional structure. Muscle regeneration therefore requires not only reestablishment of myofibers but also restoration of tissue architecture. Resident muscle stem cells (SCs) are essential for regeneration, but how SCs regenerate muscle architecture is largely unknown. We address this problem using genetic labeling of mouse SCs and whole-mount imaging to reconstruct, in three dimensions, muscle regeneration. Unexpectedly, we found that myofibers form via two distinct phases of fusion and the residual basement membrane of necrotic myofibers is critical for promoting fusion and orienting regenerated myofibers. Furthermore, the centralized myonuclei characteristic of regenerated myofibers are associated with myofibrillogenesis and endure months post injury. Finally, we elucidate two cellular mechanisms for the formation of branched myofibers, a pathology characteristic of diseased muscle. We provide a synthesis of the cellular events of regeneration and show that these differ from those used during development.
Keywords: basement membrane; fusion; macrophage; muscle; muscle stem cells; regeneration; satellite cells.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Hawke TJ, and Garry DJ (2001). Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91, 534–551. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

