Nesprin-2 is a novel scaffold protein for telethonin and FHL-2 in the cardiomyocyte sarcomere
- PMID: 38569934
- PMCID: PMC11078644
- DOI: 10.1016/j.jbc.2024.107254
Nesprin-2 is a novel scaffold protein for telethonin and FHL-2 in the cardiomyocyte sarcomere
Abstract
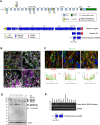
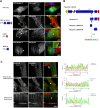
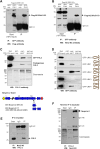
Nesprins comprise a family of multi-isomeric scaffolding proteins, forming the linker of nucleoskeleton-and-cytoskeleton complex with lamin A/C, emerin and SUN1/2 at the nuclear envelope. Mutations in nesprin-1/-2 are associated with Emery-Dreifuss muscular dystrophy (EDMD) with conduction defects and dilated cardiomyopathy (DCM). We have previously observed sarcomeric staining of nesprin-1/-2 in cardiac and skeletal muscle, but nesprin function in this compartment remains unknown. In this study, we show that specific nesprin-2 isoforms are highly expressed in cardiac muscle and localize to the Z-disc and I band of the sarcomere. Expression of GFP-tagged nesprin-2 giant spectrin repeats 52 to 53, localized to the sarcomere of neonatal rat cardiomyocytes. Yeast two-hybrid screening of a cardiac muscle cDNA library identified telethonin and four-and-half LIM domain (FHL)-2 as potential nesprin-2 binding partners. GST pull-down and immunoprecipitation confirmed the individual interactions between nesprin-2/telethonin and nesprin-2/FHL-2, and showed that nesprin-2 and telethonin binding was dependent on telethonin phosphorylation status. Importantly, the interactions between these binding partners were impaired by mutations in nesprin-2, telethonin, and FHL-2 identified in EDMD with DCM and hypertrophic cardiomyopathy patients. These data suggest that nesprin-2 is a novel sarcomeric scaffold protein that may potentially participate in the maintenance and/or regulation of sarcomeric organization and function.
Keywords: FHL-2; cardiomyopathies; interaction; nesprin-2; sarcomere organization; telethonin.
Crown Copyright © 2024. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Zhang Q., Skepper J.N., Yang F., Davies J.D., Hegyi L., Roberts R.G., et al. Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J. Cell Sci. 2001;114:4485–4498. - PubMed
-
- Zhou C., Rao L., Shanahan C.M., Zhang Q. Nesprin-1/2: roles in nuclear envelope organisation, myogenesis and muscle disease. Biochem. Soc. Trans. 2018;46:311–320. - PubMed
-
- Zhang Q., Bethmann C., Worth N.F., Davies J.D., Wasner C., Feuer A., et al. Nesprin-1 and -2 are involved in the pathogenesis of emery dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum. Mol. Genet. 2007;16:2816–2833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

