Nobiletin alleviates cisplatin-induced ototoxicity via activating autophagy and inhibiting NRF2/GPX4-mediated ferroptosis
- PMID: 38570541
- PMCID: PMC10991266
- DOI: 10.1038/s41598-024-55614-4
Nobiletin alleviates cisplatin-induced ototoxicity via activating autophagy and inhibiting NRF2/GPX4-mediated ferroptosis
Abstract
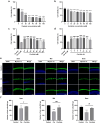
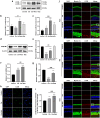
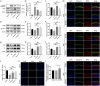
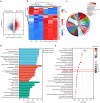
Nobiletin, a citrus polymethoxy flavonoid with antiapoptotic and antioxidative properties, could safeguard against cisplatin-induced nephrotoxicity and neurotoxicity. Cisplatin, as the pioneer of anti-cancer drug, the severe ototoxicity limits its clinical applications, while the effect of nobiletin on cisplatin-induced ototoxicity has not been identified. The current study investigated the alleviating effect of nobiletin on cisplatin-induced ototoxicity and the underlying mechanisms. Apoptosis and ROS formation were evaluated using the CCK-8 assay, Western blotting, and immunofluorescence, indicating that nobiletin attenuated cisplatin-induced apoptosis and oxidative stress. LC3B and SQSTM1/p62 were determined by Western blotting, qPCR, and immunofluorescence, indicating that nobiletin significantly activated autophagy. Nobiletin promoted the nuclear translocation of NRF2 and the transcription of its target genes, including Hmox1, Nqo1, and ferroptosis markers (Gpx4, Slc7a11, Fth, and Ftl), thereby inhibiting ferroptosis. Furthermore, RNA sequencing analysis verified that autophagy, ferroptosis, and the NRF2 signaling pathway served as crucial points for the protection of nobiletin against ototoxicity caused by cisplatin. Collectively, these results indicated, for the first time, that nobiletin alleviated cisplatin-elicited ototoxicity through suppressing apoptosis and oxidative stress, which were attributed to the activation of autophagy and the inhibition of NRF2/GPX4-mediated ferroptosis. Our study suggested that nobiletin could be a prospective agent for preventing cisplatin-induced hearing loss.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Smoorenburg, G. F., De Groot, J., Hamers, F. P. T. & Klis, S. F. L. in Ototoxicity: Basic Science and Clinical Applications Vol. 884 Annals of the New York Academy of Sciences (eds D. Henderson et al.) 192–210 (New York Acad Sciences, 1999).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

