Proliferation-driven mechanical compression induces signalling centre formation during mammalian organ development
- PMID: 38570617
- PMCID: PMC11482733
- DOI: 10.1038/s41556-024-01380-4
Proliferation-driven mechanical compression induces signalling centre formation during mammalian organ development
Abstract
Localized sources of morphogens, called signalling centres, play a fundamental role in coordinating tissue growth and cell fate specification during organogenesis. However, how these signalling centres are established in tissues during embryonic development is still unclear. Here we show that the main signalling centre orchestrating development of rodent incisors, the enamel knot (EK), is specified by a cell proliferation-driven buildup in compressive stresses (mechanical pressure) in the tissue. Direct mechanical measurements indicate that the stresses generated by cell proliferation are resisted by the surrounding tissue, creating a circular pattern of mechanical anisotropy with a region of high compressive stress at its centre that becomes the EK. Pharmacological inhibition of proliferation reduces stresses and suppresses EK formation, and application of external pressure in proliferation-inhibited conditions rescues the formation of the EK. Mechanical information is relayed intracellularly through YAP protein localization, which is cytoplasmic in the region of compressive stress that establishes the EK and nuclear in the stretched anisotropic cells that resist the pressure buildup around the EK. Together, our data identify a new role for proliferation-driven mechanical compression in the specification of a model signalling centre during mammalian organ development.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Figures




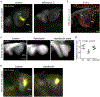








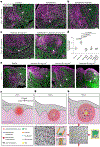
References
-
- Lecuit T & Le Goff L Orchestrating size and shape during morphogenesis. Nature 450, 189–192 (2007). - PubMed
-
- Gilmour D, Rembold M & Leptin M From morphogen to morphogenesis and back. Nature 541, 311–320 (2017). - PubMed
-
- Gurdon JB & Bourillot PY Morphogen gradient interpretation. Nature 413, 797–803 (2001). - PubMed
-
- Spemann H & Mangold H Induction of embryonic primordia by implantation of organizers from a different species. 1923. Int. J. Dev. Biol 45, 13–38 (2003). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

