Heat flows enrich prebiotic building blocks and enhance their reactivity
- PMID: 38570715
- PMCID: PMC10990939
- DOI: 10.1038/s41586-024-07193-7
Heat flows enrich prebiotic building blocks and enhance their reactivity
Abstract
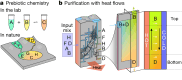
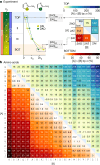
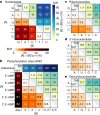
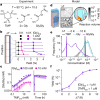
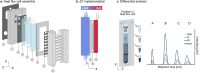
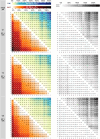
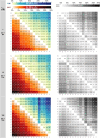
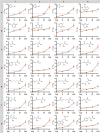
The emergence of biopolymer building blocks is a crucial step during the origins of life1-6. However, all known formation pathways rely on rare pure feedstocks and demand successive purification and mixing steps to suppress unwanted side reactions and enable high product yields. Here we show that heat flows through thin, crack-like geo-compartments could have provided a widely available yet selective mechanism that separates more than 50 prebiotically relevant building blocks from complex mixtures of amino acids, nucleobases, nucleotides, polyphosphates and 2-aminoazoles. Using measured thermophoretic properties7,8, we numerically model and experimentally prove the advantageous effect of geological networks of interconnected cracks9,10 that purify the previously mixed compounds, boosting their concentration ratios by up to three orders of magnitude. The importance for prebiotic chemistry is shown by the dimerization of glycine11,12, in which the selective purification of trimetaphosphate (TMP)13,14 increased reaction yields by five orders of magnitude. The observed effect is robust under various crack sizes, pH values, solvents and temperatures. Our results demonstrate how geologically driven non-equilibria could have explored highly parallelized reaction conditions to foster prebiotic chemistry.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Trapp O, Teichert J, Kruse F. Direct prebiotic pathway to DNA nucleosides. Angew. Chem. Int. Ed. Engl. 2019;58:9944–9947. - PubMed
-
- Becker S, et al. Unified prebiotically plausible synthesis of pyrimidine and purine RNA ribonucleotides. Science. 2019;366:76–82. - PubMed
-
- Preiner M, et al. A hydrogen-dependent geochemical analogue of primordial carbon and energy metabolism. Nat. Ecol. Evol. 2020;4:534–542. - PubMed
-
- Jash B, Tremmel P, Jovanovic D, Richert C. Single nucleotide translation without ribosomes. Nat. Chem. 2021;13:751–757. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

