Status epilepticus in patients with brain tumors and metastases: A multicenter cohort study of 208 patients and literature review
- PMID: 38570823
- PMCID: PMC10993483
- DOI: 10.1186/s42466-024-00314-7
Status epilepticus in patients with brain tumors and metastases: A multicenter cohort study of 208 patients and literature review
Abstract
Objective: Brain tumors and metastases account for approximately 10% of all status epilepticus (SE) cases. This study described the clinical characteristics, treatment, and short- and long-term outcomes of this population.
Methods: This retrospective, multi-center cohort study analyzed all brain tumor patients treated for SE at the university hospitals of Frankfurt and Marburg between 2011 and 2017.
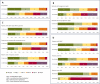
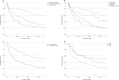
Results: The 208 patients (mean 61.5 ± 14.7 years of age; 51% male) presented with adult-type diffuse gliomas (55.8%), metastatic entities (25.5%), intracranial extradural tumors (14.4%), or other tumors (4.3%). The radiological criteria for tumor progression were evidenced in 128 (61.5%) patients, while 57 (27.4%) were newly diagnosed with tumor at admission and 113 (54.3%) had refractory SE. The mean hospital length of stay (LOS) was 14.8 days (median 12.0, range 1-57), 171 (82.2%) patients required intensive care (mean LOS 8.9 days, median 5, range 1-46), and 44 (21.2%) were administered mechanical ventilation. All patients exhibited significant functional status decline (modified Rankin Scale) post-SE at discharge (p < 0.001). Mortality at discharge was 17.3% (n = 36), with the greatest occurring in patients with metastatic disease (26.4%, p = 0.031) and those that met the radiological criteria for tumor progression (25%, p < 0.001). Long-term mortality at one year (65.9%) was highest in those diagnosed with adult-type diffuse gliomas (68.1%) and metastatic disease (79.2%). Refractory status epilepticus cases showed lower survival rates than non-refractory SE patients (log-rank p = 0.02) and those with signs of tumor progression (log-rank p = 0.001).
Conclusions: SE occurrence contributed to a decline in functional status in all cases, regardless of tumor type, tumor progression status, and SE refractoriness, while long-term mortality was increased in those with malignant tumor entities, tumor progressions, and refractory SE. SE prevention may preserve functional status and improve survival in individuals with brain tumors.
Keywords: Astrocytoma; Epilepsy; Glioblastoma; Meningioma; Seizure.
© 2024. The Author(s).
Conflict of interest statement
S. Knake has received support from Angelini Pharma, Bial, Desitin Arzneimittel, Eisai, Epilog, Jazz (GW) Pharmaceuticals, Merck Serono and UCB (Zogenix) Pharma in the form of speaker honoraria; M.W. Ronellenfitsch has received support in the form of a research grant from UCB and an honorarium for advisory board participation from Alexion; L. Habermehl has received support from Jazz Pharma, Zogenix, and Angelini pharma in the form of reimbursed travelling expenses and training costs; S. Adeberg has received support from, Accuray Inc., AstraZeneca GmbH, Bristol Myers Squibb GmbH & Co., MSD, Novocure GmbH, Merck KGaA, and Fakultät Heidelberg in the form of honoraria /travel reimbursements; research cooperations with Novocure GmbH, and scientific advisory compensation from Accuray Inc., Sanofi Genzyme, Novartis, and Novocure GmbH; M. Sebastian has received support in the form of an institutional grant from AstraZeneca of the United Kingdom, travel grants from Takeda and Pfizer of Germany, and consulting fees and honoraria for lectures from AstraZeneca, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Lilly, Roche, Boehringer Ingelheim, Amgen, Takeda, Johnson, Merck-Serono, GlaxoSmithKline, and Daiichi; Ch. Nimsky has received support as a scientific consultant for Brainlab and speaker honoraria from B. Braun, BK medical, and Brainlab. L. Timmermann has received support in the form of occasional consultation payments from Boston Scientific and speaker’s honoraria for symposia sponsored by Boston Scientific, AbbVIE, Novartis, Neuraxpharm, Teva, the Movement Disorders Society, and DIAPLAN. The institution of L.T., and not L.T. personally, received funding from Boston Scientific, the German Research Foundation, the German Ministry of Education and Research, the Otto-Loewi-Foundation, and the Deutsche Parkinson Vereinigung. Neither L.T. nor any member of his family holds stocks, stock options, patents, or financial interests in any of the above-mentioned companies or their competitors. L. Timmermann serves as the president of the German Neurological Society without payment or income. C. Grefkes reports that he is part of the editorial board of Neurological Research and Practice. J.P. Steinbach has received support in the form of honoraria for lectures, as well as advisory board participation compensation, consulting fees, and travel grants from Abbvie, Roche, Boehringer, Bristol-Myers Squibb, Medac, Mundipharma, or UCB; F. Rosenow has received support in the form of personal fees and non-financial support from UCB Pharma; personal fees from Angelini Pharma, Desitin Pharma, Eisai GMBH, Jazz Pharma, LMU Munich, Medilearn India, and Roche Pharma; and research grants from the German Research Foundation (DFG), BMBF—ERAPerMed Programme, European Union (FP7), Hessisches Ministerium für Wissenschaft und Kunst (LOEWE-Programme), Hessisches Ministerium für Soziales und Integration Detlev-Wrobel-Fonds for Epilepsy Research Frankfurt, Reiss-Stiftung, Dr. Senckenbergische-Stiftung, Ernst Max von Grunelius-Stiftung, Chaja Foundation, Dr. Schär Deutschland GmbH, Vitaflo Deutschland GmbH, Nutricia Milupa GmbH, and Desitin Pharma, outside of the submitted work; FR reports that he is part of the editorial board of Neurological Research and Practice. L. Kämppi has received support in the form of speaker honoraria from UCB and Merck and Eisai, congress/travel support from UCB and Angelini Pharma, and personal grants from the Michael Foundation, Finnish Neurology Association, and HUS Neurocenter; A. Strzelczyk has received support from Angelini Pharma, Biocodex, Desitin Arzneimittel, Eisai, Jazz (GW) Pharmaceuticals, Marinus Pharma, Precisis, Takeda, UCB (Zogenix) Pharma, and UNEEG Medical in the form of personal fees and grants. AS reports that he is part of the editorial board of Neurological Research and Practice. The remaining authors have no conflicts of interest.
Figures
Similar articles
-
Association Between Treatment Progression, Disease Refractoriness, and Burden of Illness Among Hospitalized Patients With Status Epilepticus.JAMA Neurol. 2021 May 1;78(5):588-595. doi: 10.1001/jamaneurol.2021.0520. JAMA Neurol. 2021. PMID: 33818596 Free PMC article.
-
Costs and cost-driving factors for acute treatment of adults with status epilepticus: A multicenter cohort study from Germany.Epilepsia. 2016 Dec;57(12):2056-2066. doi: 10.1111/epi.13584. Epub 2016 Oct 18. Epilepsia. 2016. PMID: 27753082
-
Costs and cost-driving factors of acute treatment of status epilepticus in children and adolescents: A cohort study from Germany.Seizure. 2022 Apr;97:63-72. doi: 10.1016/j.seizure.2022.03.014. Epub 2022 Mar 19. Seizure. 2022. PMID: 35344919
-
Treatment of status epilepticus with zonisamide: A multicenter cohort study of 34 patients and review of literature.Epilepsy Behav. 2020 Aug;109:107139. doi: 10.1016/j.yebeh.2020.107139. Epub 2020 May 14. Epilepsy Behav. 2020. PMID: 32417381 Review.
-
Efficacy and safety of perampanel in refractory and super-refractory status epilepticus: cohort study of 81 patients and literature review.J Neurol. 2021 Oct;268(10):3744-3757. doi: 10.1007/s00415-021-10506-9. Epub 2021 Mar 22. J Neurol. 2021. PMID: 33754209 Review.
Cited by
-
Early rehabilitation and improved outcomes in patients with status epilepticus: Evidence from cases presenting to the emergency department.Epilepsia Open. 2025 Apr;10(2):549-556. doi: 10.1002/epi4.70017. Epub 2025 Feb 28. Epilepsia Open. 2025. PMID: 40019491 Free PMC article.
References
-
- Olafsson E, Ludvigsson P, Hesdorffer D, Kjartansson O, Hauser WA, Gudmundsson G. Incidence of unprovoked seizures and epilepsy in Iceland and assessment of the epilepsy syndrome classification: A prospective study. The Lancet Neurology. 2005;4(10):627–634. doi: 10.1016/s1474-4422(05)70172-1. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
