PGC-1α regulates the interplay between oxidative stress, senescence and autophagy in the ageing retina important in age-related macular degeneration
- PMID: 38571282
- PMCID: PMC10992479
- DOI: 10.1111/jcmm.18051
PGC-1α regulates the interplay between oxidative stress, senescence and autophagy in the ageing retina important in age-related macular degeneration
Abstract
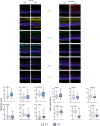
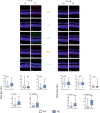
We previously showed that mice with knockout in the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PPARGC1A) gene encoding the PGC-1α protein, and nuclear factor erythroid 2 like 2 (NFE2L2) gene, exhibited some features of the age-related macular degeneration (AMD) phenotype. To further explore the mechanism behind the involvement of PGC-1α in AMD pathogenesis we used young (3-month) and old (12-month) mice with knockout in the PPARGC1A gene and age-matched wild-type (WT) animals. An immunohistochemical analysis showed age-dependent different expression of markers of oxidative stress defence, senescence and autophagy in the retinal pigment epithelium of KO animals as compared with their WT counterparts. Multivariate inference testing showed that senescence and autophagy proteins had the greatest impact on the discrimination between KO and WT 3-month animals, but proteins of antioxidant defence also contributed to that discrimination. A bioinformatic analysis showed that PGC-1α might coordinate the interplay between genes encoding proteins involved in antioxidant defence, senescence and autophagy in the ageing retina. These data support importance of PGC-1α in AMD pathogenesis and confirm the utility of mice with PGC-1α knockout as an animal model to study AMD pathogenesis.
Keywords: AMD; PGC‐1α; ageing retina; age‐related macular degeneration; autophagy; cellular senescence; oxidative stress; peroxisome proliferator‐activated receptor gamma coactivator 1‐alpha.
© 2024 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest of any other conflict associated with this manuscript.
Figures




Similar articles
-
Potential of Telomerase in Age-Related Macular Degeneration-Involvement of Senescence, DNA Damage Response and Autophagy and a Key Role of PGC-1α.Int J Mol Sci. 2021 Jul 3;22(13):7194. doi: 10.3390/ijms22137194. Int J Mol Sci. 2021. PMID: 34281248 Free PMC article. Review.
-
PGC-1α Protects RPE Cells of the Aging Retina against Oxidative Stress-Induced Degeneration through the Regulation of Senescence and Mitochondrial Quality Control. The Significance for AMD Pathogenesis.Int J Mol Sci. 2018 Aug 7;19(8):2317. doi: 10.3390/ijms19082317. Int J Mol Sci. 2018. PMID: 30087287 Free PMC article. Review.
-
Pgc-1α repression and high-fat diet induce age-related macular degeneration-like phenotypes in mice.Dis Model Mech. 2018 Aug 16;11(9):dmm032698. doi: 10.1242/dmm.032698. Dis Model Mech. 2018. PMID: 29925537 Free PMC article.
-
Loss of NRF-2 and PGC-1α genes leads to retinal pigment epithelium damage resembling dry age-related macular degeneration.Redox Biol. 2019 Jan;20:1-12. doi: 10.1016/j.redox.2018.09.011. Epub 2018 Sep 14. Redox Biol. 2019. PMID: 30253279 Free PMC article.
-
Epithelial-Mesenchymal Transition and Senescence in the Retinal Pigment Epithelium of NFE2L2/PGC-1α Double Knock-Out Mice.Int J Mol Sci. 2021 Feb 8;22(4):1684. doi: 10.3390/ijms22041684. Int J Mol Sci. 2021. PMID: 33567500 Free PMC article.
Cited by
-
Microgliosis, neuronal death, minor behavioral abnormalities and reduced endurance performance in alpha-ketoglutarate dehydrogenase complex deficient mice.Redox Biol. 2025 Sep;85:103743. doi: 10.1016/j.redox.2025.103743. Epub 2025 Jun 27. Redox Biol. 2025. PMID: 40609475 Free PMC article.
-
Sex-dependent regulation of retinal pigment epithelium and retinal function by Pgc-1α.Front Cell Neurosci. 2024 Sep 2;18:1442079. doi: 10.3389/fncel.2024.1442079. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39285939 Free PMC article.
-
Targeting shared pathways in tauopathies and age-related macular degeneration: implications for novel therapies.Front Aging Neurosci. 2024 Apr 3;16:1371745. doi: 10.3389/fnagi.2024.1371745. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38633983 Free PMC article. Review.
-
Modeling aging and retinal degeneration with mitochondrial DNA mutation burden.Aging Cell. 2024 Nov;23(11):e14282. doi: 10.1111/acel.14282. Epub 2024 Aug 29. Aging Cell. 2024. PMID: 39210608 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

