Babaodan overcomes cisplatin resistance in cholangiocarcinoma via inhibiting YAP1
- PMID: 38571483
- PMCID: PMC10997361
- DOI: 10.1080/13880209.2024.2331060
Babaodan overcomes cisplatin resistance in cholangiocarcinoma via inhibiting YAP1
Abstract
Context: Cholangiocarcinoma with highly heterogeneous, aggressive, and multidrug resistance has a poor prognosis. Although babaodan (BBD) combined with cisplatin improved non-small cell lung cancer efficacy, its impact on overcoming resistance in cholangiocarcinoma remains unexplored.
Objective: This study explored the role and mechanism of BBD on cisplatin resistance in cholangiocarcinoma cells (CCAs).
Materials and methods: Cisplatin-resistant CCAs were exposed to varying concentrations of cisplatin (25-400 μg/mL) or BBD (0.25-1.00 mg/mL) for 48 h. IC50 values, inhibition ratios, apoptosis levels, DNA damage, glutathione (GSH) levels, oxidized forms of GSH, total GSH content, and glutaminase relative activity were evaluated using the cell counting kit 8, flow cytometry, comet assay, and relevant assay kits.
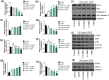
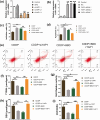
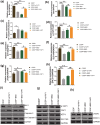
Results: BBD-reduced the cisplatin IC50 in CCAs from 118.8 to 61.83 μg/mL, leading to increased inhibition rate, apoptosis, and DNA damage, and decreased expression of B-cell lymphoma-2, p-Yes-associated protein 1/Yes-associated protein 1, solute carrier family 1 member 5, activating transcription factor 4, and ERCC excision repair 1 in a dose-dependent manner with maximum reductions of 78.97%, 51.98%, 54.03%, 56.59%, and 63.22%, respectively; bcl2-associated X and gamma histone levels were increased by 0.43-115.77% and 22.15-53.39%. The impact of YAP1 knockdown on cisplatin-resistant CCAs resembled BBD. GSH, oxidized GSH species, total GSH content, and glutaminase activity in cisplatin-resistant CCAs with BBD treatment also decreased, while YAP1 overexpression countered BBD's effects.
Discussion and conclusion: This study provides a scientific basis for BBD clinical application and provides a new direction for BBD biological mechanism research.
Keywords: Apoptosis; DNA damage; glutathione; yes-associated protein 1.
Conflict of interest statement
The authors report there are no competing interests to declare.
Figures



Similar articles
-
Lovastatin overcomes gefitinib resistance through TNF-α signaling in human cholangiocarcinomas with different LKB1 statuses in vitro and in vivo.Oncotarget. 2015 Sep 15;6(27):23857-73. doi: 10.18632/oncotarget.4408. Oncotarget. 2015. PMID: 26160843 Free PMC article.
-
Blockade of chloride channel-3 enhances cisplatin sensitivity of cholangiocarcinoma cells though inhibiting autophagy.Can J Physiol Pharmacol. 2022 Jul 1;100(7):584-593. doi: 10.1139/cjpp-2022-0058. Epub 2022 Apr 12. Can J Physiol Pharmacol. 2022. PMID: 35413227
-
Cisplatin Disrupts Proteasome Bounce-Back Effect through Suppressing ZEB1/Nfe2l1 in Cholangiocarcinoma.Front Biosci (Landmark Ed). 2024 Mar 15;29(3):106. doi: 10.31083/j.fbl2903106. Front Biosci (Landmark Ed). 2024. PMID: 38538281
-
Targeting cancer stem cells in cholangiocarcinoma (Review).Int J Oncol. 2020 Aug;57(2):397-408. doi: 10.3892/ijo.2020.5074. Epub 2020 May 28. Int J Oncol. 2020. PMID: 32468022 Free PMC article. Review.
-
Cholangiocarcinoma Therapeutics: An Update.Curr Cancer Drug Targets. 2021;21(6):457-475. doi: 10.2174/1568009621666210204152028. Curr Cancer Drug Targets. 2021. PMID: 33563168 Review.
Cited by
-
A bird's eye view of mitochondrial unfolded protein response in cancer: mechanisms, progression and further applications.Cell Death Dis. 2024 Sep 11;15(9):667. doi: 10.1038/s41419-024-07049-y. Cell Death Dis. 2024. PMID: 39261452 Free PMC article. Review.
-
Tailoring traditional Chinese medicine in cancer therapy.Mol Cancer. 2025 Jan 21;24(1):27. doi: 10.1186/s12943-024-02213-6. Mol Cancer. 2025. PMID: 39838407 Free PMC article. Review.
-
COPB2 promotes hepatocellular carcinoma progression through regulation of YAP1 nuclear translocation.Oncol Res. 2025 Mar 19;33(4):975-988. doi: 10.32604/or.2025.058085. eCollection 2025. Oncol Res. 2025. PMID: 40191726 Free PMC article.
References
-
- Alam M, Alam S, Shamsi A, Adnan M, Elasbali AM, Al-Soud WA, Alreshidi M, Hawsawi YM, Tippana A, Pasupuleti VR, et al. . 2022. Bax/Bcl-2 Cascade is regulated by the EGFR pathway: therapeutic targeting of non-small cell lung cancer. Front Oncol. 12:869672. doi: 10.3389/fonc.2022.869672. - DOI - PMC - PubMed
-
- Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, et al. . 2020. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 17(9):557–588. doi: 10.1038/s41575-020-0310-z. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
