Formoxyboranes as hydroborane surrogates for the catalytic reduction of carbonyls through transfer hydroboration
- PMID: 38571548
- PMCID: PMC10987016
- DOI: 10.1039/d3cy01702h
Formoxyboranes as hydroborane surrogates for the catalytic reduction of carbonyls through transfer hydroboration
Abstract
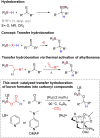
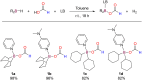
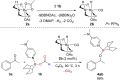
A new class of Lewis base stabilized formoxyboranes demonstrates the feasibility of catalytic transfer hydroboration. In the presence of a ruthenium catalyst, they have shown broad applicability for reducing carbonyl compounds. Various borylated alcohols are obtained in high selectivity and yields up to 99%, tolerating several functional groups. Computational studies enabled to propose a mechanism for this transformation, revealing the role of the ruthenium catalyst and the absence of hydroborane intermediates.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Yamakawa T. Masaki M. Nohira H. Bull. Chem. Soc. Jpn. 1991;64:2730–2734. doi: 10.1246/bcsj.64.2730. - DOI
- Prasad A. S. B. Kanth J. V. B. Periasamy M. Tetrahedron. 1992;48:4623–4628. doi: 10.1016/S0040-4020(01)81236-9. - DOI
- Brown H. C. Kanth J. V. B. Zaidlewicz M. J. Org. Chem. 1998;63:5154–5163. doi: 10.1021/jo980362d. - DOI
- Brown H. C. Ravindran N. J. Am. Chem. Soc. 1976;98:1785–1798. doi: 10.1021/ja00423a025. - DOI
- Chakraborty S. Zhang J. Krause J. A. Guan H. J. Am. Chem. Soc. 2010;132:8872–8873. doi: 10.1021/ja103982t. - DOI - PubMed
- Chong C. C. Kinjo R. ACS Catal. 2015;5:3238–3259. doi: 10.1021/acscatal.5b00428. - DOI
- Barger C. J. Motta A. Weidner V. L. Lohr T. L. Marks T. J. ACS Catal. 2019;9:9015–9024. doi: 10.1021/acscatal.9b02605. - DOI
-
- Brotherton R. J., Weber C. J., Guibert C. R. and Little J. L., Boron compounds, in Ullmann's encyclopedia of industrial chemistry, John Wiley & Sons, Ltd, Ed., 2000, https://onlinelibrary.wiley.com/doi/10.1002/14356007.a04_309
-
- Studer A. Amrein S. Angew. Chem., Int. Ed. 2000;39:3080–3082. doi: 10.1002/1521-3773(20000901)39:17<3080::AID-ANIE3080>3.0.CO;2-E. - DOI - PubMed
- Amrein S. Timmermann A. Studer A. Org. Lett. 2001;3:2357–2360. doi: 10.1021/ol016160c. - DOI - PubMed
- Amrein S. Studer A. Helv. Chim. Acta. 2002;85:3559–3574. doi: 10.1002/1522-2675(200210)85:10<3559::AID-HLCA3559>3.0.CO;2-6. - DOI
- Simonneau A. Oestreich M. Angew. Chem., Int. Ed. 2013;52:11905–11907. doi: 10.1002/anie.201305584. - DOI - PubMed
- Keess S. Simonneau A. Oestreich M. Organometallics. 2015;34:790–799. doi: 10.1021/om501284a. - DOI
- Chauvier C. Thuery P. Cantat T. Angew. Chem., Int. Ed. 2016;55:14096–14100. doi: 10.1002/anie.201607201. - DOI - PubMed
-
- Wang D. Astruc D. Chem. Rev. 2015;115:6621–6686. doi: 10.1021/acs.chemrev.5b00203. - DOI - PubMed
- Nie R. F. Tao Y. W. Nie Y. Q. Lu T. L. Wang J. S. Zhang Y. S. Lu X. Y. Xu C. C. ACS Catal. 2021;11:1071–1095. doi: 10.1021/acscatal.0c04939. - DOI
- Wei D. Sang R. Sponholz P. Junge H. Beller M. Nat. Energy. 2022;7:438–447. doi: 10.1038/s41560-022-01019-4. - DOI
-
- Farlow M. W. Adkins H. J. Am. Chem. Soc. 2002;57:2222–2223. doi: 10.1021/ja01314a054. - DOI
- Graf E. Leitner W. J. Chem. Soc., Chem. Commun. 1992:623–624. doi: 10.1039/C39920000623. - DOI
- Jessop P. G. Ikariya T. Noyori R. Chem. Rev. 1995;95:259–272. doi: 10.1021/cr00034a001. - DOI
- Wang W. H. Himeda Y. Muckerman J. T. Manbeck G. F. Fujita E. Chem. Rev. 2015;115:12936–12973. doi: 10.1021/acs.chemrev.5b00197. - DOI - PubMed
- Kang P. Cheng C. Chen Z. Schauer C. K. Meyer T. J. Brookhart M. J. Am. Chem. Soc. 2012;134:5500–5503. doi: 10.1021/ja300543s. - DOI - PubMed
- Chen L. Guo Z. Wei X. G. Gallenkamp C. Bonin J. Anxolabehere-Mallart E. Lau K. C. Lau T. C. Robert M. J. Am. Chem. Soc. 2015;137:10918–10921. doi: 10.1021/jacs.5b06535. - DOI - PubMed
- Taheri A. Berben L. A. Chem. Commun. 2016;52:1768–1777. doi: 10.1039/C5CC09041E. - DOI - PubMed
- Francke R. Schille B. Roemelt M. Chem. Rev. 2018;118:4631–4701. doi: 10.1021/acs.chemrev.7b00459. - DOI - PubMed
- Bullock R. M. Das A. K. Appel A. M. Chem. – Eur. J. 2017;23:7626–7641. doi: 10.1002/chem.201605066. - DOI - PubMed
- Gao S. Lin Y. Jiao X. Sun Y. Luo Q. Zhang W. Li D. Yang J. Xie Y. Nature. 2016;529:68–71. doi: 10.1038/nature16455. - DOI - PubMed
LinkOut - more resources
Full Text Sources
