Establishment of a graphene quantum dot (GQD) based steroid binding assay for the nuclear progesterone receptor (pgr)
- PMID: 38571552
- PMCID: PMC10987840
- DOI: 10.1016/j.bbrep.2024.101691
Establishment of a graphene quantum dot (GQD) based steroid binding assay for the nuclear progesterone receptor (pgr)
Abstract
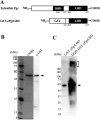
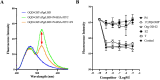
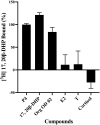
Previously, we established a homogeneous assay for membrane progesterone receptor alpha (mPRα) ligands by conjugating semiconductor nanoparticles known as graphene quantum dots (GQDs) to mPRα. When mixed with a progesterone-BSA-fluorescein isothiocyanate conjugate (P4-BSA-FITC), fluorescence occurred by fluorescence resonance energy transfer (FRET) but was reduced by the ligand-receptor binding activity. The established way showed ligand specificity as mPRα protein. In this study, we tried to establish the same way for nuclear progesterone receptor (Pgr). The ligand-binding domain (LBD) of zebrafish Pgr (zPgrLBD) was expressed as a fusion protein with glutathione S-transferase (GST) (GST-zPgrLBD). The recombinant protein was then purified and coupled with GQDs to produce GQD-conjugated GST-zPgrLBD (GQD-GST-zPgrLBD). When mixed with a P4-BSA-FITC and activated by 370 nm light, fluorescence at 520 nm appeared by FRET mechanism. Fluorescence at 520 nm was reduced by adding free progesterone to the reaction mixture. Reduction of fluorescence was induced by zPgr ligands but not by steroids or chemicals that do not interact with zPgr. The results showed the formation of a complex of GQD-GST-zPgrLBD and P4-BSA-FITC with ligand-receptor binding. The binding of the compounds was further confirmed by a radiolabeled steroid binding assay. A homogenous ligand-binding assay for nuclear progesterone receptor has been established.
Keywords: FRET; Graphene quantum dots; Nuclear progesterone receptor; Progesterone; Steroids.
© 2024 The Authors.
Conflict of interest statement
The authors declare that there no conflicts of interest.
Figures
Similar articles
-
Establishment of a steroid binding assay for goldfish membrane progesterone receptor (mPR) by coupling with graphene quantum dots (GQDs).Fish Physiol Biochem. 2024 Jun;50(3):1331-1339. doi: 10.1007/s10695-024-01315-8. Epub 2024 Feb 8. Fish Physiol Biochem. 2024. PMID: 38329580
-
Establishment of a steroid binding assay for membrane progesterone receptor alpha (PAQR7) by using graphene quantum dots (GQDs).Biochem Biophys Res Commun. 2022 Feb 12;592:1-6. doi: 10.1016/j.bbrc.2022.01.002. Epub 2022 Jan 5. Biochem Biophys Res Commun. 2022. PMID: 35007844
-
Evidence of binding between diethylstilbestrol (DES) and the goldfish (Carassius auratus) membrane progesterone receptor α.Toxicol Mech Methods. 2024 Jun;34(5):563-571. doi: 10.1080/15376516.2024.2311185. Epub 2024 Feb 5. Toxicol Mech Methods. 2024. PMID: 38317456
-
Comparison between steroid binding to membrane progesterone receptor alpha (mPRalpha) and to nuclear progesterone receptor: correlation with physicochemical properties assessed by comparative molecular field analysis and identification of mPRalpha-specific agonists.Steroids. 2010 Apr;75(4-5):314-22. doi: 10.1016/j.steroids.2010.01.010. Epub 2010 Jan 22. Steroids. 2010. PMID: 20096719 Free PMC article.
-
An all-graphene quantum dot Förster resonance energy transfer (FRET) probe for ratiometric detection of HE4 ovarian cancer biomarker.Colloids Surf B Biointerfaces. 2021 Feb;198:111458. doi: 10.1016/j.colsurfb.2020.111458. Epub 2020 Nov 12. Colloids Surf B Biointerfaces. 2021. PMID: 33246782
References
-
- Garg D., Ng S.S.M., Baig K.M., Driggers P., Segars J. Progesterone-mediated non-classical signaling. Trends Endocrinol. Metabol. 2017;28:656–668. - PubMed
-
- Morel Y., Roucher F., Plotton I., Goursaud C., Tardy V., Mallet D. Evolution of steroids during pregnancy: maternal, placental and fetal synthesis, Annales d'endocrinologie. Elsevier. 2016:82–89. - PubMed
-
- Pepe G.J., Albrecht E.D. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr. Rev. 1995;16:608–648. - PubMed
-
- Brisken C. Progesterone signalling in breast cancer: a neglected hormone coming into the limelight. Nat. Rev. Cancer. 2013;13:385–396. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

