Treatment intensification with bempedoic acid to achieve LDL-C goal in patients with ASCVD: A simulation model using a real-world patient cohort in the US
- PMID: 38571880
- PMCID: PMC10987878
- DOI: 10.1016/j.athplu.2024.01.006
Treatment intensification with bempedoic acid to achieve LDL-C goal in patients with ASCVD: A simulation model using a real-world patient cohort in the US
Abstract
Background and aims: Guidelines recommend that high-risk patients with atherosclerotic cardiovascular disease (ASCVD) be treated with maximally tolerated statins to lower low-density lipoprotein cholesterol (LDL-C) levels and reduce the risk of major adverse cardiovascular events. In patients whose LDL-C remains elevated, non-statin adjunct therapies, including ezetimibe (EZE), bempedoic acid (BA), and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors are recommended.
Methods: The impact of BA and EZE in a fixed-dose combination (FDC) on LDL-C goal attainment was evaluated using a simulation model developed for a United States cohort of high-risk adults with ASCVD. Treatment was simulated for 73,056 patients not at goal (LDL-C >70 mg/dL), comparing BA + EZE (FDC), EZE only, and no oral adjunct therapy (NOAT). The addition of PCSK9 inibitors was assumed after 1 year in patients not at LDL-C goal. Treatment efficacy was estimated from clinical trials. Patient-level outcomes were predicted over a 10-year horizon accounting for treatment discontinuation and general mortality.
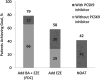
Results: Baseline mean age of the cohort was 67 years, most were White (79%) and male (56%). A majority had established coronary artery disease (75%), 48% had diabetes, and mean LDL-C was 103.0 mg/dL. After 1 year, 79% of patients achieved LDL-C goal (mean, 61.1 mg/dL) with BA + EZE (FDC) compared to 58% and 42% with EZE (71.7 mg/dL) and NOAT (78.4 mg/dL), respectively.
Conclusions: This simulation shows that adding BA + EZE (FDC) to maximally tolerated statins would result in more patients achieving LDL-C goal than adding EZE alone or NOAT.
© 2024 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Kristen Migliaccio-Walle reports financial support was provided by Esperion Therapeutics Inc. Kristen Migliaccio-Walle reports writing assistance was provided by Spark Therapeutics Inc. David Elsea, Kristel Griffith, Rajshree Pandey reports financial support was provided by Esperion Therapeutics Inc. Anand Gupta reports financial support was provided by Esperion Therapeutics Inc. Evelyn Sarnes, Kristin Gillard reports writing assistance was provided by Spark Therapeutics Inc. Evelyn Sarnes, Kristin Gillard reports a relationship with Esperion Therapeutics Inc that includes: employment and equity or stocks. co-author previously employed by Genetech Inc - RP.
Figures

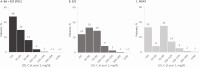
References
-
- Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. 2019. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
