Recent progress of polymeric microneedle-assisted long-acting transdermal drug delivery
- PMID: 38571937
- PMCID: PMC10987780
- DOI: 10.3389/jpps.2024.12434
Recent progress of polymeric microneedle-assisted long-acting transdermal drug delivery
Erratum in
-
Corrigendum: Recent progress of polymeric microneedle-assisted long-acting transdermal drug delivery.J Pharm Pharm Sci. 2025 Jan 27;28:14083. doi: 10.3389/jpps.2025.14083. eCollection 2025. J Pharm Pharm Sci. 2025. PMID: 39935622 Free PMC article.
Abstract
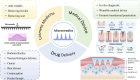
Microneedle (MN)-assisted drug delivery technology has gained increasing attention over the past two decades. Its advantages of self-management and being minimally invasive could allow this technology to be an alternative to hypodermic needles. MNs can penetrate the stratum corneum and deliver active ingredients to the body through the dermal tissue in a controlled and sustained release. Long-acting polymeric MNs can reduce administration frequency to improve patient compliance and therapeutic outcomes, especially in the management of chronic diseases. In addition, long-acting MNs could avoid gastrointestinal reactions and reduce side effects, which has potential value for clinical application. In this paper, advances in design strategies and applications of long-acting polymeric MNs are reviewed. We also discuss the challenges in scale manufacture and regulations of polymeric MN systems. These two aspects will accelerate the effective clinical translation of MN products.
Keywords: drug delivery systems; long-acting drug release; microneedles; polymeric; transdermal.
Copyright © 2024 Meng, Qiao, Xin, Ju and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Recent Advances in Polymer Microneedles for Drug Transdermal Delivery: Design Strategies and Applications.Macromol Rapid Commun. 2022 Apr;43(8):e2200037. doi: 10.1002/marc.202200037. Epub 2022 Mar 23. Macromol Rapid Commun. 2022. PMID: 35286762 Review.
-
Microneedle-mediated transdermal drug delivery for treating diverse skin diseases.Acta Biomater. 2021 Feb;121:119-133. doi: 10.1016/j.actbio.2020.12.004. Epub 2020 Dec 5. Acta Biomater. 2021. PMID: 33285323 Review.
-
Current trends in polymer microneedle for transdermal drug delivery.Int J Pharm. 2020 Sep 25;587:119673. doi: 10.1016/j.ijpharm.2020.119673. Epub 2020 Jul 30. Int J Pharm. 2020. PMID: 32739388 Free PMC article. Review.
-
Microneedle array systems for long-acting drug delivery.Eur J Pharm Biopharm. 2021 Feb;159:44-76. doi: 10.1016/j.ejpb.2020.12.006. Epub 2020 Dec 24. Eur J Pharm Biopharm. 2021. PMID: 33359666 Review.
-
Long-acting microneedle formulations.Adv Drug Deliv Rev. 2023 Oct;201:115055. doi: 10.1016/j.addr.2023.115055. Epub 2023 Aug 17. Adv Drug Deliv Rev. 2023. PMID: 37597586 Review.
Cited by
-
Microneedle-Assisted Delivery of Curcumin: Evaluating the Effects of Needle Length and Formulation.Micromachines (Basel). 2025 Jan 29;16(2):155. doi: 10.3390/mi16020155. Micromachines (Basel). 2025. PMID: 40047600 Free PMC article.
-
Polymeric microneedle advancements in macromolecule drug delivery: current trends, challenges, and future perspectives.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr 17. doi: 10.1007/s00210-025-04117-8. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40244451 Review.
-
Transdermal Semaglutide Administration in Mice: Reduces Body Weight by Suppressing Appetite and Enhancing Metabolic Rate.Biology (Basel). 2025 May 20;14(5):575. doi: 10.3390/biology14050575. Biology (Basel). 2025. PMID: 40427764 Free PMC article.
-
Bioengineered microneedles and nanomedicine as therapeutic platform for tissue regeneration.J Nanobiotechnology. 2025 Aug 19;23(1):573. doi: 10.1186/s12951-025-03623-4. J Nanobiotechnology. 2025. PMID: 40830894 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

