Identifying antinuclear antibody positive individuals at risk for developing systemic autoimmune disease: development and validation of a real-time risk model
- PMID: 38571954
- PMCID: PMC10987951
- DOI: 10.3389/fimmu.2024.1384229
Identifying antinuclear antibody positive individuals at risk for developing systemic autoimmune disease: development and validation of a real-time risk model
Abstract
Objective: Positive antinuclear antibodies (ANAs) cause diagnostic dilemmas for clinicians. Currently, no tools exist to help clinicians interpret the significance of a positive ANA in individuals without diagnosed autoimmune diseases. We developed and validated a risk model to predict risk of developing autoimmune disease in positive ANA individuals.
Methods: Using a de-identified electronic health record (EHR), we randomly chart reviewed 2,000 positive ANA individuals to determine if a systemic autoimmune disease was diagnosed by a rheumatologist. A priori, we considered demographics, billing codes for autoimmune disease-related symptoms, and laboratory values as variables for the risk model. We performed logistic regression and machine learning models using training and validation samples.
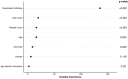
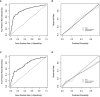
Results: We assembled training (n = 1030) and validation (n = 449) sets. Positive ANA individuals who were younger, female, had a higher titer ANA, higher platelet count, disease-specific autoantibodies, and more billing codes related to symptoms of autoimmune diseases were all more likely to develop autoimmune diseases. The most important variables included having a disease-specific autoantibody, number of billing codes for autoimmune disease-related symptoms, and platelet count. In the logistic regression model, AUC was 0.83 (95% CI 0.79-0.86) in the training set and 0.75 (95% CI 0.68-0.81) in the validation set.
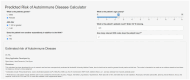
Conclusion: We developed and validated a risk model that predicts risk for developing systemic autoimmune diseases and can be deployed easily within the EHR. The model can risk stratify positive ANA individuals to ensure high-risk individuals receive urgent rheumatology referrals while reassuring low-risk individuals and reducing unnecessary referrals.
Keywords: antinuclear antibodies; autoimmune disease; electronic health record; rheumatology; risk model.
Copyright © 2024 Barnado, Moore, Domenico, Green, Camai, Suh, Han, Walker, Anderson, Caruth, Katta, McCoy and Byrne.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The outcome of children referred to a pediatric rheumatology clinic with a positive antinuclear antibody test but without an autoimmune disease.Pediatrics. 1995 Jun;95(6):892-5. Pediatrics. 1995. PMID: 7761217
-
Rule-based and machine learning algorithms identify patients with systemic sclerosis accurately in the electronic health record.Arthritis Res Ther. 2019 Dec 30;21(1):305. doi: 10.1186/s13075-019-2092-7. Arthritis Res Ther. 2019. PMID: 31888720 Free PMC article.
-
[Investigation of the Contribution of Autoantibodies to Clinical Diagnosis in Liver Pathologies and the Identification of Accompanying Autoimmune Diseases].Mikrobiyol Bul. 2022 Jan;56(1):81-94. doi: 10.5578/mb.20229907. Mikrobiyol Bul. 2022. PMID: 35088962 Turkish.
-
The Clinical Relevance of Anti-DFS70 Autoantibodies.Clin Rev Allergy Immunol. 2017 Apr;52(2):202-216. doi: 10.1007/s12016-016-8564-5. Clin Rev Allergy Immunol. 2017. PMID: 27350273 Review.
-
Clinical utility of antinuclear antibody tests in children.BMC Pediatr. 2004 Jul 9;4:13. doi: 10.1186/1471-2431-4-13. BMC Pediatr. 2004. PMID: 15245579 Free PMC article. Review.
Cited by
-
Emerging Mechanisms and Biomarkers Associated with T-Cells and B-Cells in Autoimmune Disorders.Clin Rev Allergy Immunol. 2025 Feb 11;68(1):14. doi: 10.1007/s12016-025-09022-9. Clin Rev Allergy Immunol. 2025. PMID: 39932617 Review.
-
Utility of common investigations for suspected inflammatory arthritis in adults.Aust Prescr. 2024 Aug;47(4):119-124. doi: 10.18773/austprescr.2024.035. Aust Prescr. 2024. PMID: 39228464 Free PMC article. Review.
-
Role of autoimmune phenomena in nonalcoholic fatty liver disease: Insights and limitations.World J Hepatol. 2025 Mar 27;17(3):103835. doi: 10.4254/wjh.v17.i3.103835. World J Hepatol. 2025. PMID: 40177209 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

