Halofuginone-guided nano-local therapy: Nano-thermosensitive hydrogels for postoperative metastatic canine mammary carcinoma with scar removal
- PMID: 38572023
- PMCID: PMC10987322
- DOI: 10.1016/j.ijpx.2024.100241
Halofuginone-guided nano-local therapy: Nano-thermosensitive hydrogels for postoperative metastatic canine mammary carcinoma with scar removal
Abstract
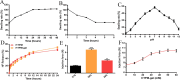
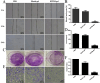
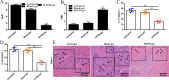
In female dogs, the highest morbidity and mortality rates cancer are the result of mammary adenocarcinoma, which presents with metastases in the lung. Other than early surgical removal, however, no special methods are available to treat mammary adenocarcinoma. Because human breast cancer and canine mammary carcinoma share clinical characteristics and heterogeneity, the canine model is a suitable spontaneous tumor model for breast cancer in humans. In this study, the physical swelling method was used to prepare halofuginone-loaded D-α-tocopherol polyethylene glycol 1000 succinate (TPGS) polymer micelles nano-thermosensitive hydrogels (HTPM-gel). Furthermore, HTPM-gel was investigated via characterization, morphology, properties such as swelling experiment and in vitro release with reflecting its splendid nature. Moreover, HTPM-gel was further examined its capability to anti-proliferation, anti-migration, and anti-invasion. Ultimately, HTPM-gel was investigated for its in vivo anticancer activity in the post-operative metastatic and angiogenic canine mammary carcinoma. HTPM-gel presented spherical under transmission electron microscope (TEM) and represented grid structure under scanning electron microscope (SEM), with hydrodynamic diameter (HD) of 20.25 ± 2.5 nm and zeta potential (ZP) of 15.10 ± 1.82 mV. Additionally, HTPM-gel own excellent properties comprised of pH-dependent swelling behavior, sustained release behavior. To impede the migration, invasion, and proliferation of CMT-U27 cells, we tested the efficacy of HTPM-gel. Evaluation of in vivo anti-tumor efficacy demonstrates HTPM-gel exhibit a splendid anti-metastasis and anti-angiogenic ability, with exhibiting ideal biocompatibility. Notably, HTPM-gel also inhibited the scar formation in the healing process after surgery. In summary, HTPM-gel exhibited anti-metastasis and anti-angiogenic and scar repair features. According to the results of this study, HTPM-gel has encouraging clinical potential to treat tumors with multifunctional hydrogel.
Keywords: Angiogenesis; Halofuginone; Metastasis; Postoperative; Scar removal; Thermosensitive hydrogels.
© 2024 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Injectable nano-in situ-thermosensitive-hydrogels based on halofuginone and silver for postoperative treatment against triple-negative breast cancer.Int J Pharm. 2024 Aug 15;661:124384. doi: 10.1016/j.ijpharm.2024.124384. Epub 2024 Jun 23. Int J Pharm. 2024. PMID: 38917957
-
Using halofuginone-silver thermosensitive nanohydrogels with antibacterial and anti-inflammatory properties for healing wounds infected with Staphylococcus aureus.Life Sci. 2024 Feb 15;339:122414. doi: 10.1016/j.lfs.2024.122414. Epub 2024 Jan 10. Life Sci. 2024. PMID: 38216121
-
Orally Administered Halofuginone-Loaded TPGS Polymeric Micelles Against Triple-Negative Breast Cancer: Enhanced Absorption and Efficacy with Reduced Toxicity and Metastasis.Int J Nanomedicine. 2022 May 30;17:2475-2491. doi: 10.2147/IJN.S352538. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35668999 Free PMC article.
-
Nano-encapsulation of halofuginone hydrobromide enhances anticoccidial activity against Eimeria tenella in chickens.Biomater Sci. 2023 Feb 28;11(5):1725-1738. doi: 10.1039/d2bm01543a. Biomater Sci. 2023. PMID: 36648120
-
Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy.Polymers (Basel). 2022 Jun 12;14(12):2379. doi: 10.3390/polym14122379. Polymers (Basel). 2022. PMID: 35745954 Free PMC article. Review.
Cited by
-
Establishment and Partial Characterization of Canine Mammary Tumor Cell Lines.Animals (Basel). 2025 Jul 7;15(13):1991. doi: 10.3390/ani15131991. Animals (Basel). 2025. PMID: 40646890 Free PMC article.
-
A Comprehensive Review of Smart Thermosensitive Nanocarriers for Precision Cancer Therapy.Int J Mol Sci. 2025 Jul 29;26(15):7322. doi: 10.3390/ijms26157322. Int J Mol Sci. 2025. PMID: 40806454 Free PMC article. Review.
References
-
- Agrawal M., Saraf S., Saraf S., Dubey S.K., Puri A., Gupta U., Kesharwani P., Ravichandiran V., Kumar P., Naidu V.G.M., Murty U.S., Ajazuddin Alexander A. Stimuli-responsive in situ gelling system for nose-to-brain drug delivery. J. Control. Release. 2020;327:235–265. - PubMed
-
- Ali I., Rizwan A., Vu T.T., Jo S.H., Oh C.W., Kim Y.H., Park S.H., Lim K.T. NIR-responsive carboxymethyl-cellulose hydrogels containing thioketal-linkages for on-demand drug delivery system. Int. J. Biol. Macromol. 2024;260(Pt 2) - PubMed
-
- Alonso-Miguel D., Valdivia G., García-San-José P., Alonso-Diez Á., Clares I., Portero M., Peña L., Pérez-Alenza M.D. Clinical outcome of dogs diagnosed with canine inflammatory mammary cancer treated with metronomic cyclophosphamide, a cyclooxygenase-2 inhibitor and toceranib phosphate. Vet. Comp. Oncol. 2022;20(1):179–188. - PubMed
-
- Altuntaş E., Yener G. Formulation and evaluation of thermoreversible in situ nasal gels containing mometasone furoate for allergic rhinitis. AAPS Pharm. Sci. Tech. 2017;18(7):2673–2682. - PubMed
-
- Augustine R., Kim D.K., Kalva N., Eom K.H., Kim J.H., Kim I. Multi-stimuli-responsive nanomicelles fabricated using synthetic polymer polylysine conjugates for tumor microenvironment dependent drug delivery. J. Mater. Chem. B. 2020;8(26):5745–5755. - PubMed
LinkOut - more resources
Full Text Sources

