Effect of level of sedation on outcomes in critically ill adult patients: a systematic review of clinical trials with meta-analysis and trial sequential analysis
- PMID: 38572080
- PMCID: PMC10990717
- DOI: 10.1016/j.eclinm.2024.102569
Effect of level of sedation on outcomes in critically ill adult patients: a systematic review of clinical trials with meta-analysis and trial sequential analysis
Abstract
Background: Sedation is routinely administered to critically ill patients to alleviate anxiety, discomfort, and patient-ventilator asynchrony. However, it must be balanced against risks such as delirium and prolonged intensive care stays. This study aimed to investigate the effects of different levels of sedation in critically ill adults.
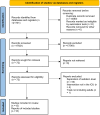
Methods: Systematic review with meta-analysis and trial sequential analysis (TSA) of randomised clinical trials including critically ill adults admitted to the intensive care unit. CENTRAL, MEDLINE, Embase, LILACS, and Web of Science were searched from their inception to 13 June 2023. Risks of bias were assessed using the Cochrane risk of bias tool. Primary outcome was all-cause mortality. Aggregate data were synthesised with meta-analyses and TSA, and the certainty of the evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach. This study is registered with PROSPERO: CRD42023386960.
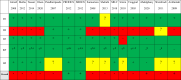
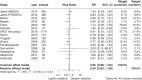
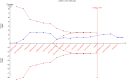
Findings: Fifteen trials randomising 4352 patients were included, of which 13 were assessed high risk of bias. Meta-analyses comparing lighter to deeper sedation showed no evidence of a difference in all-cause mortality (risk ratio (RR) 0.94, 95% confidence interval (CI) 0.83-1.06; p = 0.28; 15 trials; moderate certainty evidence), serious adverse events (RR 0.99, CI 0.92-1.06; p = 0.80; 15 trials; moderate certainty evidence), or delirium (RR 1.01, 95% CI 0.94-1.09; p = 0.78; 11 trials; moderate certainty evidence). TSA showed that when assessing mortality, a relative risk reduction of 16% or more between the compared interventions could be rejected.
Interpretation: The level of sedation has not been shown to affect the risks of death, delirium, and other serious adverse events in critically ill adult patients. While TSA suggests that additional trials are unlikely to significantly change the conclusion of the meta-analyses, the certainty of evidence was moderate. This suggests a need for future high-quality studies with higher methodological rigor.
Funding: None.
Keywords: Critically ill; Intensive care; Meta-analysis; Mortality; Sedation; Systematic review.
© 2024 The Author(s).
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: TLM declares that her institution received grants (ICECAP (UG3HL134269)) for her role as site principal investigator for SIREN funded RCT for cardiac arrest patients and are on clinical standardization committee. TLM's institution has secured funding for her involvement as a co-investigator in an ancillary study related to ICECAP, with a 5% Full-Time Equivalent support allocation (PRECICECAP (R01NS119825)). Additionally, TLM is principial investigator for a grant, reducing rural disparities in cardiac arrest outcomes by standardization of care (P20GM139745)), with 50% FTE support with funds to the institution. MSk received speakers fee 2021 and 2022 for BARD Medical (Ireland). JHä declares that she received grants from Paulo Foundation, Tor och Kirsti Johanssons Hjärt och Cancerstiftelse, Finska Läkaresällskapet, NordForsk, and Government funding for University Level research (2021, 2022, and 2023). JHä also declared that her institution (Tampere University Hospital research services) received a consultation fee from Paion and that JHä participated on Data Safety Monitoring Board or Adversary Board in Paion. JHä also received payment for lectures for Finnish Medical association, Laboratory Medicine, and Duodecim (a Finnish society of physicians). Additionally, JHä has a role in Educational Committee of Scandinavian Society of Anaesthesiology, board member of Advanced Educational Committee of Intensive Care Medicine in Scandinavian Society of Anaesthesiology and Intensive Care Medicine, and European Society of Intensive Care Medicine: National representative and faculty in CoBaTriCe Finnish Sepsis Society and have minor share of Orion B stock. MCR declare that his institution (University of Queensland) received grant support from National Health and Medical Research Council, Australia, Medical Research Future Fund, and Intensive Care Foundation, Australian Defence Force, and Royal Brisbane and Women's Hospital Foundation the past 36 months. MCR also received payment for expert testimony (in cases not related to the subject matter of this paper) from government of the Northern Territory High Court of New Zealand, received payment for being a member of DSMB for clod stored platelet trial, and his wife had stock investment in ETF that includes biomedical shares, which were sold 12 months ago. NN declared that his institution received support for the present study from Swedish Research Council and governmental funds within the Swedish Health Care (ALF). All other authors declared no conflicts of interests.
Figures

Similar articles
-
Level of sedation in critically ill adult patients: a protocol for a systematic review with meta-analysis and trial sequential analysis.BMJ Open. 2022 Sep 8;12(9):e061806. doi: 10.1136/bmjopen-2022-061806. BMJ Open. 2022. PMID: 36691212 Free PMC article.
-
Antibiotics for secondary prevention of coronary heart disease.Cochrane Database Syst Rev. 2021 Feb 23;2(2):CD003610. doi: 10.1002/14651858.CD003610.pub4. Cochrane Database Syst Rev. 2021. PMID: 33704780 Free PMC article.
-
Interventions for treatment of COVID-19: Second edition of a living systematic review with meta-analyses and trial sequential analyses (The LIVING Project).PLoS One. 2021 Mar 11;16(3):e0248132. doi: 10.1371/journal.pone.0248132. eCollection 2021. PLoS One. 2021. PMID: 33705495 Free PMC article.
-
Fever therapy in febrile adults: systematic review with meta-analyses and trial sequential analyses.BMJ. 2022 Jul 12;378:e069620. doi: 10.1136/bmj-2021-069620. BMJ. 2022. PMID: 35820685 Free PMC article.
-
Interventions for treatment of COVID-19: A living systematic review with meta-analyses and trial sequential analyses (The LIVING Project).PLoS Med. 2020 Sep 17;17(9):e1003293. doi: 10.1371/journal.pmed.1003293. eCollection 2020 Sep. PLoS Med. 2020. PMID: 32941437 Free PMC article.
Cited by
-
Impact of sedation levels on outcomes in neurocritical care patients with intracranial hemorrhage: a retrospective cohort study.Neurosurg Rev. 2025 Apr 3;48(1):351. doi: 10.1007/s10143-025-03507-z. Neurosurg Rev. 2025. PMID: 40175838
-
[S3 guideline on sepsis-prevention, diagnosis, therapy, and follow-up care-update 2025].Med Klin Intensivmed Notfmed. 2025 Aug 18. doi: 10.1007/s00063-025-01317-1. Online ahead of print. Med Klin Intensivmed Notfmed. 2025. PMID: 40824313 Review. German.
References
-
- Devlin J.W., Skrobik Y., Gelinas C., et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. - PubMed
-
- Celis-Rodríguez E., Díaz Cortés J.C., Cárdenas Bolívar Y.R., et al. Evidence-based clinical practice guidelines for the management of sedoanalgesia and delirium in critically ill adult patients. Med Intensiva. 2020;44(3):171–184. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

