β-amyloid accumulation enhances microtubule associated protein tau pathology in an APPNL-G-F/MAPTP301S mouse model of Alzheimer's disease
- PMID: 38572146
- PMCID: PMC10987964
- DOI: 10.3389/fnins.2024.1372297
β-amyloid accumulation enhances microtubule associated protein tau pathology in an APPNL-G-F/MAPTP301S mouse model of Alzheimer's disease
Abstract
Introduction: The study of the pathophysiology study of Alzheimer's disease (AD) has been hampered by lack animal models that recapitulate the major AD pathologies, including extracellular -amyloid (A) deposition, intracellular aggregation of microtubule associated protein tau (MAPT), inflammation and neurodegeneration.
Methods: The humanized APPNL-G-F knock-in mouse line was crossed to the PS19 MAPTP301S, over-expression mouse line to create the dual APPNL-G-F/PS19 MAPTP301S line. The resulting pathologies were characterized by immunochemical methods and PCR.
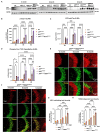
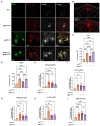
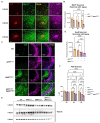
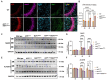
Results: We now report on a double transgenic APPNL-G-F/PS19 MAPTP301S mouse that at 6 months of age exhibits robust A plaque accumulation, intense MAPT pathology, strong inflammation and extensive neurodegeneration. The presence of A pathology potentiated the other major pathologies, including MAPT pathology, inflammation and neurodegeneration. MAPT pathology neither changed levels of amyloid precursor protein nor potentiated A accumulation. Interestingly, study of immunofluorescence in cleared brains indicates that microglial inflammation was generally stronger in the hippocampus, dentate gyrus and entorhinal cortex, which are regions with predominant MAPT pathology. The APPNL-G-F/MAPTP301S mouse model also showed strong accumulation of N6-methyladenosine (m6A), which was recently shown to be elevated in the AD brain. m6A primarily accumulated in neuronal soma, but also co-localized with a subset of astrocytes and microglia. The accumulation of m6A corresponded with increases in METTL3 and decreases in ALKBH5, which are enzymes that add or remove m6A from mRNA, respectively.
Discussion: Our understanding of the pathophysiology of Alzheimer's disease (AD) has been hampered by lack animal models that recapitulate the major AD pathologies, including extracellular -amyloid (A) deposition, intracellular aggregation of microtubule associated protein tau (MAPT), inflammation and neurodegeneration. The APPNL-G-F/MAPTP301S mouse recapitulates many features of AD pathology beginning at 6 months of aging, and thus represents a useful new mouse model for the field.
Keywords: RNA binding proteins; RNA methylation; clarity; neuritic plaques; neurodegeneration; neuropathology; tau oligomers; tauopathy.
Copyright © 2024 Jiang, Roberts, Wong, Zhang, Webber, Libera, Wang, Kilci, Jenkins, Ortiz, Dorrian, Sun, Sun, Rashad, Kornbrek, Daley, Dedon, Nguyen, Xia, Saito, Saido and Wolozin.
Conflict of interest statement
CK and BN are employed by LifeCanvas Technologies. BW is co-founder and Chief Scientific Officer for Aquinnah Pharmaceuticals Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

