Administration of adiponectin receptor agonist AdipoRon relieves cancer cachexia by mitigating inflammation in tumour-bearing mice
- PMID: 38572511
- PMCID: PMC11154773
- DOI: 10.1002/jcsm.13454
Administration of adiponectin receptor agonist AdipoRon relieves cancer cachexia by mitigating inflammation in tumour-bearing mice
Abstract
Background: Cancer cachexia is a life-threatening, inflammation-driven wasting syndrome that remains untreatable. Adiponectin, the most abundant adipokine, plays an important role in several metabolic processes as well as in inflammation modulation. Our aim was to test whether administration of AdipoRon (AR), a synthetic agonist of the adiponectin receptors, prevents the development of cancer cachexia and its related muscle atrophy.
Methods: The effect of AR on cancer cachexia was investigated in two distinct murine models of colorectal cancer. First, 7-week-old CD2F1 male mice were subcutaneously injected with colon-26 carcinoma cells (C26) or vehicle (CT). Six days after injection, mice were treated for 5 days with AdipoRon (50 mg/kg/day; C26 + AR) or the corresponding vehicle (CT and C26). Additionally, a genetic model, the ApcMin/+ mouse, that develops spontaneously numerous intestinal polyps, was used. Eight-week-old male ApcMin/+ mice were treated with AdipoRon (50 mg/kg/day; Apc + AR) or the corresponding vehicle (Apc) over a period of 12 weeks, with C57BL/6J wild-type mice used as controls. In both models, several parameters were assessed in vivo: body weight, grip strength and serum parameters, as well as ex vivo: molecular changes in muscle, fat and liver.
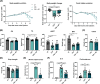
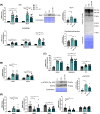
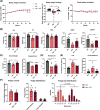
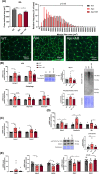
Results: The protective effect of AR on cachexia development was observed in both cachectic C26 and ApcMin/+ mice. In these mice, AR administration led to a significant alleviation of body weight loss and muscle wasting, together with rescued muscle strength (P < 0.05 for all). In both models, AR had a strong anti-inflammatory effect, reflected by lower systemic interleukin-6 levels (-55% vs. C26, P < 0.001 and -80% vs. Apc mice, P < 0.05), reduced muscular inflammation as indicated by lower levels of Socs3, phospho-STAT3 and Serpina3n, an acute phase reactant (P < 0.05 for all). In addition, AR blunted circulating levels of corticosterone (-46% vs. C26 mice, P < 0.001 and -60% vs. Apc mice, P < 0.05), the predominant murine glucocorticoid known to induce muscle atrophy. Accordingly, key glucocorticoid-responsive factors implicated in atrophy programmes were-or tended to be-significantly blunted in skeletal muscle by AR. Finally, AR protected against lipid metabolism alterations observed in ApcMin/+ mice, as it mitigated the increase in circulating triglyceride levels (-38%, P < 0.05) by attenuating hepatic triglyceride synthesis and fatty acid uptake by the liver.
Conclusions: Altogether, these results show that AdipoRon rescued the cachectic phenotype by alleviating body weight loss and muscle atrophy, along with restraining inflammation and hypercorticism in preclinical murine models. Therefore, AdipoRon could represent an innovative therapeutic strategy to counteract cancer cachexia.
Keywords: adiponectin; cachexia; cancer; inflammation; skeletal muscle.
© 2024 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Argiles JM, Busquets S, Stemmler B, Lopez‐Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014;14:754–762. - PubMed
-
- Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012;16:153–166. - PubMed
-
- Benny Klimek ME, Aydogdu T, Link MJ, Pons M, Koniaris LG, Zimmers TA. Acute inhibition of myostatin‐family proteins preserves skeletal muscle in mouse models of cancer cachexia. Biochem Biophys Res Commun 2010;391:1548–1554. - PubMed
-
- Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 2010;142:531–543. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

