Mutual regulation of CD4+ T cells and intravascular fibrin in infections
- PMID: 38572559
- PMCID: PMC11290509
- DOI: 10.3324/haematol.2023.284619
Mutual regulation of CD4+ T cells and intravascular fibrin in infections
Abstract
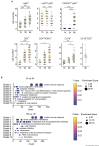
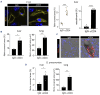
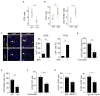
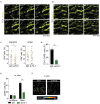
Innate myeloid cells especially neutrophils and their extracellular traps are known to promote intravascular coagulation and thrombosis formation in infections and various other conditions. Innate myeloid cell-dependent fibrin formation can support systemic immunity while its dysregulation enhances the severity of infectious diseases. Less is known about the immune mechanisms preventing dysregulation of fibrin homeostasis in infection. During experimental systemic infections local fibrin deposits in the liver microcirculation cause rapid arrest of CD4+ T cells. Arrested T-helper cells mostly represent Th17 cells that partially originate from the small intestine. Intravascular fibrin deposits activate mouse and human CD4+ T cells which can be mediated by direct fibrin-CD4+ T-cell interactions. Activated CD4+ T cells suppress fibrin deposition and microvascular thrombosis by directly counteracting coagulation activation by neutrophils and classical monocytes. T-cell activation, which is initially triggered by IL-12p40- and MHC-II-dependent mechanisms, enhances intravascular fibrinolysis via LFA-1. Moreover, CD4+ T cells disfavor the association of the thrombin-activatable fibrinolysis inhibitor (TAFI) with fibrin whereby fibrin deposition is increased by TAFI in the absence but not in the presence of T cells. In human infections thrombosis development is inversely related to microvascular levels of CD4+ T cells. Thus, fibrin promotes LFA-1-dependent T-helper cell activation in infections which drives a negative feedback cycle that rapidly restricts intravascular fibrin and thrombosis development.
Figures


References
-
- Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2018;16(2):231-241. - PubMed
-
- Van Bruggen S, Martinod K. The coming of age of neutrophil extracellular traps in thrombosis: where are we now and where are we headed? Immunol Rev. 2023;314(1):376-398. - PubMed
-
- Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13(1):34-45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

