Protein G-quadruplex interactions and their effects on phase transitions and protein aggregation
- PMID: 38572746
- PMCID: PMC11077067
- DOI: 10.1093/nar/gkae229
Protein G-quadruplex interactions and their effects on phase transitions and protein aggregation
Abstract
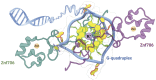
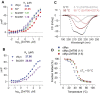
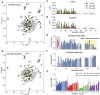
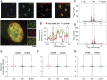
The SERF family of proteins were originally discovered for their ability to accelerate amyloid formation. Znf706 is an uncharacterized protein whose N-terminus is homologous to SERF proteins. We show here that human Znf706 can promote protein aggregation and amyloid formation. Unexpectedly, Znf706 specifically interacts with stable, non-canonical nucleic acid structures known as G-quadruplexes. G-quadruplexes can affect gene regulation and suppress protein aggregation; however, it is unknown if and how these two activities are linked. We find Znf706 binds preferentially to parallel G-quadruplexes with low micromolar affinity, primarily using its N-terminus, and upon interaction, its dynamics are constrained. G-quadruplex binding suppresses Znf706's ability to promote protein aggregation. Znf706 in conjunction with G-quadruplexes therefore may play a role in regulating protein folding. RNAseq analysis shows that Znf706 depletion specifically impacts the mRNA abundance of genes that are predicted to contain high G-quadruplex density. Our studies give insight into how proteins and G-quadruplexes interact, and how these interactions affect both partners and lead to the modulation of protein aggregation and cellular mRNA levels. These observations suggest that the SERF family of proteins, in conjunction with G-quadruplexes, may have a broader role in regulating protein folding and gene expression than previously appreciated.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



Update of
-
Protein G-quadruplex interactions and their effects on phase transitions and protein aggregation.bioRxiv [Preprint]. 2024 Mar 20:2023.09.21.558871. doi: 10.1101/2023.09.21.558871. bioRxiv. 2024. Update in: Nucleic Acids Res. 2024 May 8;52(8):4702-4722. doi: 10.1093/nar/gkae229. PMID: 37790366 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

