Characteristics and outcomes of cerebral venous thrombosis associated with COVID-19
- PMID: 38572798
- PMCID: PMC11418515
- DOI: 10.1177/23969873241241885
Characteristics and outcomes of cerebral venous thrombosis associated with COVID-19
Abstract
Introduction: Previous reports and meta-analyses derived from small case series reported a mortality rate of up to 40% in patients with coronavirus disease 2019 associated cerebral venous thrombosis (COVID-CVT). We assessed the clinical characteristics and outcomes in an international cohort of patients with COVID-CVT.
Patients and methods: This was a registry study of consecutive COVID-CVT patients diagnosed between March 2020 and March 2023. Data collected by the International Cerebral Venous Thrombosis Consortium from patients with CVT diagnosed between 2017 and 2018 served as a comparison. Outcome analyses were adjusted for age and sex.
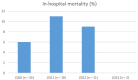
Results: We included 70 patients with COVID-CVT from 23 hospitals in 15 countries and 206 controls from 14 hospitals in 13 countries. The proportion of women was smaller in the COVID-CVT group (50% vs 68%, p < 0.01). A higher proportion of COVID-CVT patients presented with altered mental state (44% vs 25%, p < 0.01), the median thrombus load was higher in COVID-CVT patients (3 [IQR 2-4] vs 2 [1-3], p < 0.01) and the length of hospital stay was longer compared to controls (11 days [IQR 7-20] vs 8 [4-15], p = 0.02). In-hospital mortality did not differ (5/67 [7%, 95% CI 3-16] vs 7/206 [3%, 2-7], aOR 2.6 [95% CI 0.7-9]), nor did the frequency of functional independence after 6 months (modified Rankin Scale 0-2; 45/58 [78%, 95% CI 65-86] vs 161/185 [87%, 81-91], aOR 0.5 [95% CI 0.2-1.02]).
Conclusion: In contrast to previous studies, the in-hospital mortality rate and functional outcomes during follow-up did not differ between COVID-CVT patients and the pre-COVID-19 controls.
Keywords: COVID-19; Cerebral venous thrombosis; stroke.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AS reports a grant from the Swiss Heart Foundation. MRH reports grants from SITEM Research Support Funds and Swiss National Science Foundation, Swiss Heart Foundation, not directly related to this manuscript. MA reports personal fees from AstraZeneca, Bayer, Bristol Myers Squibb, Covidien, Daiichi Sankyo, Medtronic, Novartis, Pfizer, and Amgen. JMC has received grants paid to his institution from Boehringer Ingelheim and Bayer, and payments paid to his institution for data safety monitoring board participation by Bayer. JMF has received personal fees from Boehringer Ingelheim, Bayer, and Daiichi Sankyo as well as grants from Bayer. DAS reports travel support from Boehringer Ingelheim, speaker fees from Bayer, and Advisory Board participation for AstraZeneca. TT has received personal fees from Argenx, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Inventiva, and Portola Pharma. NR received consultant fees from Novartis. KJ has received academic grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG 965417) for research on CVT. SP received research support from BMS/Pfizer, Boehringer-Ingelheim, Daiichi Sankyo, European Union, German Federal Joint Committee Innovation Fund, and German Federal Ministry of Education and Research, Helena Laboratories and Werfen as well as speakers’ honoraria/consulting fees from Alexion, AstraZeneca, Bayer, Boehringer-Ingelheim, BMS/Pfizer, Daiichi Sankyo, Portola, and Werfen (all outside the submitted work). TNN reports advisory board Idorsia, Brainomix. KA reports a grant from Swiss National Science Foundation and Medtronic advisory board participation in 2022, not related to this manuscript. EL has received academic grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG 942851), Swedish Neurologic Society, Elsa and Gustav Lindh’s Foundation, Wennerströms’ Foundation, P-O Ahl’s Foundation and Rune and Ulla Amlöv’s Foundation for research on CVT. All other co-authors report no disclosures.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

