Dual and Triple Incretin-Based Co-agonists: Novel Therapeutics for Obesity and Diabetes
- PMID: 38573467
- PMCID: PMC11043266
- DOI: 10.1007/s13300-024-01566-x
Dual and Triple Incretin-Based Co-agonists: Novel Therapeutics for Obesity and Diabetes
Abstract
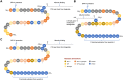
The discovery of long-acting incretin receptor agonists represents a major stride forward in tackling the dual epidemic of obesity and diabetes. Here we outline the evolution of incretin-based pharmacotherapy, from exendin-4 to the discovery of the multi-incretin hormone receptor agonists that look set to be our next step toward curing diabetes and obesity. We discuss the multiagonists currently in clinical trials and the improvement in efficacy each new generation of these drugs bring. The success of these agents in preclinical models and clinical trials suggests a promising future for multiagonists in the treatment of metabolic diseases, with the most recent glucose-dependent insulinotropic peptide receptor:glucagon-like peptide 1 receptor:glucagon receptor (GIPR:GLP-1R:GCGR) triagonists rivaling the efficacy of bariatric surgery. However, further research is needed to fully understand how these therapies exert their effect on body weight and in the last section we cover open questions about the potential mechanisms of multiagonist drugs, and the understanding of how gut-brain communication can be leveraged to achieve sustained body weight loss without adverse effects.
Keywords: Diabetes; Dual-agonist; GIP; GLP-1; Glucagon; Incretin; Obesity; Triagonist.
© 2024. The Author(s).
Conflict of interest statement
Robert M. Gutgesell and Rubén Nogueiras declare that they have nothing to disclose. MHT is a member of the scientific advisory board of ERX Pharmaceuticals, Cambridge, Mass. He was a member of the Research Cluster Advisory Panel (ReCAP) of the Novo Nordisk Foundation between 2017 and 2019. He attended a scientific advisory board meeting of the Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, in 2016. He received funding for his research projects by Novo Nordisk (2016–2020) and Sanofi-Aventis (2012–2019). He was a consultant for Bionorica SE (2013–2017), Menarini Ricerche S.p.A. (2016), and Bayer Pharma AG Berlin (2016). As former Director of the Helmholtz Diabetes Center and the Institute for Diabetes and Obesity at Helmholtz Zentrum München (2011–2018), and since 2018, as CEO of Helmholtz Zentrum München, he has been responsible for collaborations with a multitude of companies and institutions, worldwide. In this capacity, he discussed potential projects with and has signed/signs contracts for his institute(s) and for the staff for research funding and/or collaborations with industry and academia, worldwide, including but not limited to pharmaceutical corporations like Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Medigene, Arbormed, BioSyngen, and others. In this role, he was/is further responsible for commercial technology transfer activities of his institute(s), including diabetes-related patent portfolios of Helmholtz Zentrum München as, e.g., WO/2016/188932 A2 or WO/2017/194499 A1. MHT confirms that to the best of his knowledge none of the above funding sources were involved in the preparation of this paper. TDM receives research funding from Novo Nordisk but these funds are unrelated to the work described here. TDM received speaking fees within the last 3 years from Novo Nordisk, Eli Lilly, AstraZeneca, Merck, Berlin Chemie AG, and Mercodia.
Figures

References
-
- IDF Diabetes Atlas 10th edition. International Diabetes Federation; 2021. (IDF Diabetes Atlas). Report no. 10. https://diabetesatlas.org/atlas/tenth-edition/.
-
- Lobstein T, Brinsden H, Neveux M. World Obesity Atlas 2022. Ludgate House, 107–111 Fleet Street, London, EC4A 2AB: World Obesity; 2022 May. https://www.worldobesity.org/resources/resource-library/world-obesity-at....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

