Clinical Value of Molecular Targets and FDA-Approved Genome-Targeted Cancer Therapies
- PMID: 38573645
- PMCID: PMC11099684
- DOI: 10.1001/jamaoncol.2024.0194
Clinical Value of Molecular Targets and FDA-Approved Genome-Targeted Cancer Therapies
Abstract
Importance: The number of new genome-targeted cancer drugs has increased, offering the possibility of personalized therapy, often at a very high cost.
Objective: To assess the validity of molecular targets and therapeutic benefits of US Food and Drug Administration-approved genome-targeted cancer drugs based on the outcomes of their corresponding pivotal clinical trials.
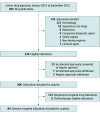
Design and settings: In this cohort study, all genome-targeted cancer drugs that were FDA-approved between January 1, 2015, and December 31, 2022, were analyzed. From FDA drug labels and trial reports, key characteristics of pivotal trials were extracted, including the outcomes assessed.
Main outcomes and measures: The strength of evidence supporting molecular targetability was assessed using the European Society for Medical Oncology (ESMO) Scale for Clinical Actionability of Molecular Targets (ESCAT). Clinical benefit for their approved indications was evaluated using the ESMO-Magnitude of Clinical Benefit Scale (ESMO-MCBS). Substantial clinical benefit was defined as a grade of A or B for curative intent and 4 or 5 for noncurative intent. Molecular targets qualifying for ESCAT category level I-A and I-B associated with substantial clinical benefit by ESMO-MCBS were rated as high-benefit genomic-based cancer treatments.
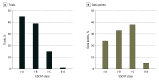
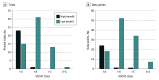
Results: A total of 50 molecular-targeted drugs covering 84 indications were analyzed. Forty-five indications (54%) were approved based on phase 1 or phase 2 pivotal trials, 45 (54%) were supported by single-arm pivotal trials, and 48 (57%) were approved on the basis of subgroup analyses. By each indication, 46 of 84 primary end points (55%) were overall response rate (median [IQR] overall response rate, 57% [40%-69%]; median [IQR] duration of response, 11.1 [9.2-19.8] months). Among the 84 pivotal trials supporting these 84 indications, 38 trials (45%) had I-A ESCAT targetability, and 32 (38%) had I-B targetability. Overall, 24 of 84 trials (29%) demonstrated substantial clinical benefit via ESMO-MCBS. Combining these ratings, 24 of 84 indications (29%) were associated with high-benefit genomic-based cancer treatments.
Conclusions and relevance: The results of this cohort study demonstrate that among recently approved molecular-targeted cancer therapies, fewer than one-third demonstrated substantial patient benefits at approval. Benefit frameworks such as ESMO-MCBS and ESCAT can help physicians, patients, and payers identify therapies with the greatest clinical potential.
Conflict of interest statement
Figures
Similar articles
-
Predictors of withdrawal of anticancer drug indications granted accelerated approval: a retrospective cohort study.EClinicalMedicine. 2025 May 31;84:103088. doi: 10.1016/j.eclinm.2025.103088. eCollection 2025 Jun. EClinicalMedicine. 2025. PMID: 40687736 Free PMC article.
-
Factors in Time to Full Approval or Withdrawal for Anticancer Medicines Granted Accelerated Approval by the FDA.JAMA Netw Open. 2025 Mar 3;8(3):e252026. doi: 10.1001/jamanetworkopen.2025.2026. JAMA Netw Open. 2025. PMID: 40136298 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
Mechanisms and Assessment of Genotoxicity of Metallic Engineered Nanomaterials in the Human Environment.Biomedicines. 2024 Oct 20;12(10):2401. doi: 10.3390/biomedicines12102401. Biomedicines. 2024. PMID: 39457713 Free PMC article. Review.
-
Predictors of withdrawal of anticancer drug indications granted accelerated approval: a retrospective cohort study.EClinicalMedicine. 2025 May 31;84:103088. doi: 10.1016/j.eclinm.2025.103088. eCollection 2025 Jun. EClinicalMedicine. 2025. PMID: 40687736 Free PMC article.
-
Cancer Drugs Approved Based on Surrogate Endpoint: A Retrospective Observational Study in the United States and China.Cancer Med. 2025 Apr;14(8):e70864. doi: 10.1002/cam4.70864. Cancer Med. 2025. PMID: 40230311 Free PMC article.
-
Improving the Dosing Schedules of Targeted Anticancer Agents.Pharmaceuticals (Basel). 2025 Jun 6;18(6):848. doi: 10.3390/ph18060848. Pharmaceuticals (Basel). 2025. PMID: 40573244 Free PMC article. Review.
-
Factors in Time to Full Approval or Withdrawal for Anticancer Medicines Granted Accelerated Approval by the FDA.JAMA Netw Open. 2025 Mar 3;8(3):e252026. doi: 10.1001/jamanetworkopen.2025.2026. JAMA Netw Open. 2025. PMID: 40136298 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

