Proteomics identifies complement protein signatures in patients with alcohol-associated hepatitis
- PMID: 38573776
- PMCID: PMC11141929
- DOI: 10.1172/jci.insight.174127
Proteomics identifies complement protein signatures in patients with alcohol-associated hepatitis
Abstract
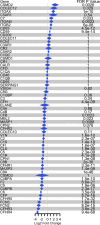
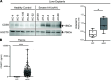
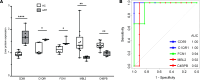
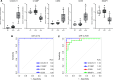
Diagnostic challenges continue to impede development of effective therapies for successful management of alcohol-associated hepatitis (AH), creating an unmet need to identify noninvasive biomarkers for AH. In murine models, complement contributes to ethanol-induced liver injury. Therefore, we hypothesized that complement proteins could be rational diagnostic/prognostic biomarkers in AH. Here, we performed a comparative analysis of data derived from human hepatic and serum proteome to identify and characterize complement protein signatures in severe AH (sAH). The quantity of multiple complement proteins was perturbed in liver and serum proteome of patients with sAH. Multiple complement proteins differentiated patients with sAH from those with alcohol cirrhosis (AC) or alcohol use disorder (AUD) and healthy controls (HCs). Serum collectin 11 and C1q binding protein were strongly associated with sAH and exhibited good discriminatory performance among patients with sAH, AC, or AUD and HCs. Furthermore, complement component receptor 1-like protein was negatively associated with pro-inflammatory cytokines. Additionally, lower serum MBL associated serine protease 1 and coagulation factor II independently predicted 90-day mortality. In summary, meta-analysis of proteomic profiles from liver and circulation revealed complement protein signatures of sAH, highlighting a complex perturbation of complement and identifying potential diagnostic and prognostic biomarkers for patients with sAH.
Keywords: Complement; Diagnostics; Hepatitis; Hepatology.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK061732/DK/NIDDK NIH HHS/United States
- U01 AA026933/AA/NIAAA NIH HHS/United States
- BX003259-05A1/US Department of Veterans Office of Research & Development
- U01 AA021890/AA/NIAAA NIH HHS/United States
- U01 AA026977/AA/NIAAA NIH HHS/United States
- U01 AA026980/AA/NIAAA NIH HHS/United States
- R21 AA028117/AA/NIAAA NIH HHS/United States
- U01 AA026976/AA/NIAAA NIH HHS/United States
- K08 AA028794/AA/NIAAA NIH HHS/United States
- I01 BX003259/BX/BLRD VA/United States
- R01 DK113196/DK/NIDDK NIH HHS/United States
- R01 DK134675/DK/NIDDK NIH HHS/United States
- P50 AA024333/AA/NIAAA NIH HHS/United States
- R56 HL141744/HL/NHLBI NIH HHS/United States
- U01 AA021893/AA/NIAAA NIH HHS/United States
- U01 AA021901/AA/NIAAA NIH HHS/United States
- P20 GM113226/GM/NIGMS NIH HHS/United States
- U01 DK062470/DK/NIDDK NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- R01 AA016399/AA/NIAAA NIH HHS/United States
- U01 AA026962/AA/NIAAA NIH HHS/United States
- R01 GM119174/GM/NIGMS NIH HHS/United States
- P50 AA024337/AA/NIAAA NIH HHS/United States
- R21 AA026398/AA/NIAAA NIH HHS/United States
- U01 AA026975/AA/NIAAA NIH HHS/United States
- U01 AA021918/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

