Activating SRC/MAPK signaling via 5-HT1A receptor contributes to the effect of vilazodone on improving thrombocytopenia
- PMID: 38573820
- PMCID: PMC10994662
- DOI: 10.7554/eLife.94765
Activating SRC/MAPK signaling via 5-HT1A receptor contributes to the effect of vilazodone on improving thrombocytopenia
Abstract
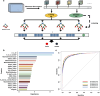
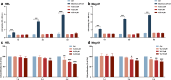
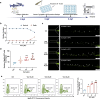
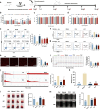
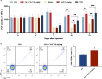
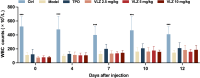
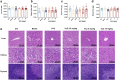
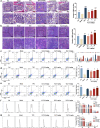
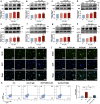
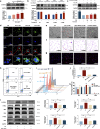
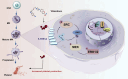
Thrombocytopenia caused by long-term radiotherapy and chemotherapy exists in cancer treatment. Previous research demonstrates that 5-Hydroxtrayptamine (5-HT) and its receptors induce the formation of megakaryocytes (MKs) and platelets. However, the relationships between 5-HT1A receptor (5-HTR1A) and MKs is unclear so far. We screened and investigated the mechanism of vilazodone as a 5-HTR1A partial agonist in promoting MK differentiation and evaluated its therapeutic effect in thrombocytopenia. We employed a drug screening model based on machine learning (ML) to screen the megakaryocytopoiesis activity of Vilazodone (VLZ). The effects of VLZ on megakaryocytopoiesis were verified in HEL and Meg-01 cells. Tg (itga2b: eGFP) zebrafish was performed to analyze the alterations in thrombopoiesis. Moreover, we established a thrombocytopenia mice model to investigate how VLZ administration accelerates platelet recovery and function. We carried out network pharmacology, Western blot, and immunofluorescence to demonstrate the potential targets and pathway of VLZ. VLZ has been predicted to have a potential biological action. Meanwhile, VLZ administration promotes MK differentiation and thrombopoiesis in cells and zebrafish models. Progressive experiments showed that VLZ has a potential therapeutic effect on radiation-induced thrombocytopenia in vivo. The network pharmacology and associated mechanism study indicated that SRC and MAPK signaling are both involved in the processes of megakaryopoiesis facilitated by VLZ. Furthermore, the expression of 5-HTR1A during megakaryocyte differentiation is closely related to the activation of SRC and MAPK. Our findings demonstrated that the expression of 5-HTR1A on MK, VLZ could bind to the 5-HTR1A receptor and further regulate the SRC/MAPK signaling pathway to facilitate megakaryocyte differentiation and platelet production, which provides new insights into the alternative therapeutic options for thrombocytopenia.
Keywords: 5-HT1A receptor; medicine; megakaryocyte; mouse; thrombocytopenia; vilazodone.
© 2024, Zhou, Ni, Liao et al.
Conflict of interest statement
LZ, CN, RL, XT, TY, MR, MH, RL, XZ, DQ, LW, FH, XX, YW, JL, YW, JW No competing interests declared
Figures


Update of
- doi: 10.1101/2023.12.16.571981
- doi: 10.7554/eLife.94765.1
- doi: 10.7554/eLife.94765.2
Similar articles
-
Novel chemical-structure TPOR agonist, TMEA, promotes megakaryocytes differentiation and thrombopoiesis via mTOR and ERK signalings.Phytomedicine. 2023 Feb;110:154637. doi: 10.1016/j.phymed.2022.154637. Epub 2022 Dec 28. Phytomedicine. 2023. PMID: 36610353
-
Multimodal discovery of bavachinin A: A natural FLT3 agonist for treating thrombocytopenia.Phytomedicine. 2025 May;140:156597. doi: 10.1016/j.phymed.2025.156597. Epub 2025 Mar 1. Phytomedicine. 2025. PMID: 40058315
-
Discovery of a novel megakaryopoiesis enhancer, ingenol, promoting thrombopoiesis through PI3K-Akt signaling independent of thrombopoietin.Pharmacol Res. 2022 Mar;177:106096. doi: 10.1016/j.phrs.2022.106096. Epub 2022 Jan 22. Pharmacol Res. 2022. PMID: 35077844
-
Megakaryocyte modification of platelets in thrombocytopenia.Curr Opin Hematol. 2018 Sep;25(5):410-415. doi: 10.1097/MOH.0000000000000451. Curr Opin Hematol. 2018. PMID: 29985173 Review.
-
Linkage between the mechanisms of thrombocytopenia and thrombopoiesis.Blood. 2016 Mar 10;127(10):1234-41. doi: 10.1182/blood-2015-07-607903. Epub 2016 Jan 19. Blood. 2016. PMID: 26787737 Free PMC article. Review.
Cited by
-
Rivaroxaban Ameliorates Sunitinib-Induced Injury of Cardiomyocytes via Repressing MAPK Signaling Pathway.Cardiovasc Ther. 2025 Jul 25;2025:2208110. doi: 10.1155/cdr/2208110. eCollection 2025. Cardiovasc Ther. 2025. PMID: 40755506 Free PMC article.
-
Regulating mitochondrial oxidative phosphorylation and MAPK signaling: wedelolactone as a novel therapeutic for radiation-induced thrombocytopenia.Front Pharmacol. 2025 Apr 30;16:1508215. doi: 10.3389/fphar.2025.1508215. eCollection 2025. Front Pharmacol. 2025. PMID: 40371333 Free PMC article.
-
Targeting CCR5 with miltefosine as a therapeutic strategy for thrombocytopenia.iScience. 2025 Apr 8;28(5):112379. doi: 10.1016/j.isci.2025.112379. eCollection 2025 May 16. iScience. 2025. PMID: 40292315 Free PMC article.
References
-
- Barnes NM, Ahern GP, Becamel C, Bockaert J, Camilleri M, Chaumont-Dubel S, Claeysen S, Cunningham KA, Fone KC, Gershon M, Di Giovanni G, Goodfellow NM, Halberstadt AL, Hartley RM, Hassaine G, Herrick-Davis K, Hovius R, Lacivita E, Lambe EK, Leopoldo M, Levy FO, Lummis SCR, Marin P, Maroteaux L, McCreary AC, Nelson DL, Neumaier JF, Newman-Tancredi A, Nury H, Roberts A, Roth BL, Roumier A, Sanger GJ, Teitler M, Sharp T, Villalón CM, Vogel H, Watts SW, Hoyer D. International union of basic and clinical pharmacology. CX. Classification of receptors for 5-hydroxytryptamine; Pharmacology and function. Pharmacological Reviews. 2021;73:310–520. doi: 10.1124/pr.118.015552. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
Grants and funding
- 82074129/National Natural Science Foundation of China
- 82374073/National Natural Science Foundation of China
- 82004073/National Natural Science Foundation of China
- 2022ZYD0087/Natural Science Foundation of Sichuan Province
- 2022YFS0607/Natural Science Foundation of Sichuan Province
- 22ZYZYTS0191/Natural Science Foundation of Sichuan Province
- 2022YFS0614/Natural Science Foundation of Sichuan Province
- 2022JDJQ0061/Natural Science Foundation of Sichuan Province
- 2020LZXNYDZ03/Science and Technology Planning Project of Luzhou City
- 2020LZXNYDP01/Science and Technology Planning Project of Luzhou City
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

