Selection, engineering, and in vivo testing of a human leukocyte antigen-independent T-cell receptor recognizing human mesothelin
- PMID: 38574067
- PMCID: PMC10994368
- DOI: 10.1371/journal.pone.0301175
Selection, engineering, and in vivo testing of a human leukocyte antigen-independent T-cell receptor recognizing human mesothelin
Abstract
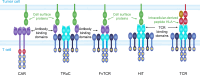
Background: Canonical α/β T-cell receptors (TCRs) bind to human leukocyte antigen (HLA) displaying antigenic peptides to elicit T cell-mediated cytotoxicity. TCR-engineered T-cell immunotherapies targeting cancer-specific peptide-HLA complexes (pHLA) are generating exciting clinical responses, but owing to HLA restriction they are only able to target a subset of antigen-positive patients. More recently, evidence has been published indicating that naturally occurring α/β TCRs can target cell surface proteins other than pHLA, which would address the challenges of HLA restriction. In this proof-of-concept study, we sought to identify and engineer so-called HLA-independent TCRs (HiTs) against the tumor-associated antigen mesothelin.
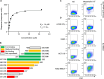
Methods: Using phage display, we identified a HiT that bound well to mesothelin, which when expressed in primary T cells, caused activation and cytotoxicity. We subsequently engineered this HiT to modulate the T-cell response to varying levels of mesothelin on the cell surface.
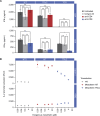
Results: The isolated HiT shows cytotoxic activity and demonstrates killing of both mesothelin-expressing cell lines and patient-derived xenograft models. Additionally, we demonstrated that HiT-transduced T cells do not require CD4 or CD8 co-receptors and, unlike a TCR fusion construct, are not inhibited by soluble mesothelin. Finally, we showed that HiT-transduced T cells are highly efficacious in vivo, completely eradicating xenografted human solid tumors.
Conclusion: HiTs can be isolated from fully human TCR-displaying phage libraries against cell surface-expressed antigens. HiTs are able to fully activate primary T cells both in vivo and in vitro. HiTs may enable the efficacy seen with pHLA-targeting TCRs in solid tumors to be translated to cell surface antigens.
Copyright: © 2024 Hiscox et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
This study was funded by Adaptimmune. Adaptimmune contributed to study design, data analysis, decision to publish, and preparation of the manuscript. Writing and editorial support was funded by Adaptimmune. All authors are employees of Adaptimmune or were at the time of the study. Authors may hold stock in Adaptimmune. This does not alter our adherence to PLOS ONE policies on sharing data and materials
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

