Sexual dimorphism in skin immunity is mediated by an androgen-ILC2-dendritic cell axis
- PMID: 38574174
- PMCID: PMC12086714
- DOI: 10.1126/science.adk6200
Sexual dimorphism in skin immunity is mediated by an androgen-ILC2-dendritic cell axis
Abstract
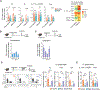
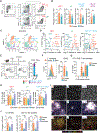
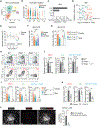
Males and females exhibit profound differences in immune responses and disease susceptibility. However, the factors responsible for sex differences in tissue immunity remain poorly understood. Here, we uncovered a dominant role for type 2 innate lymphoid cells (ILC2s) in shaping sexual immune dimorphism within the skin. Mechanistically, negative regulation of ILC2s by androgens leads to a reduction in dendritic cell accumulation and activation in males, along with reduced tissue immunity. Collectively, our results reveal a role for the androgen-ILC2-dendritic cell axis in controlling sexual immune dimorphism. Moreover, this work proposes that tissue immune set points are defined by the dual action of sex hormones and the microbiota, with sex hormones controlling the strength of local immunity and microbiota calibrating its tone.
Conflict of interest statement
Figures


Comment in
-
Sex differences in tissue immunity.Science. 2024 Apr 12;384(6692):159-160. doi: 10.1126/science.ado8542. Epub 2024 Apr 4. Science. 2024. PMID: 38574173
References
-
- Klein SL, Flanagan KL, Sex differences in immune responses. Nature Reviews Immunology 16, 626–638 (2016). - PubMed
-
- Chen W, Mempel M, Traidl-Hofmann C, Al Khusaei S, Ring J, Gender aspects in skin diseases. J. Eur. Acad. Dermatol. Venereol 24, 1378–1385 (2010). - PubMed
-
- Mohammad I et al., Estrogen receptor α contributes to T cell–mediated autoimmune inflammation by promoting T cell activation and proliferation. Science signaling 11, eaap9415 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

