Electronic Patient-Reported Outcome-Based Symptom Management Versus Usual Care After Lung Cancer Surgery: Long-Term Results of a Multicenter, Randomized, Controlled Trial
- PMID: 38574304
- PMCID: PMC11191061
- DOI: 10.1200/JCO.23.01854
Electronic Patient-Reported Outcome-Based Symptom Management Versus Usual Care After Lung Cancer Surgery: Long-Term Results of a Multicenter, Randomized, Controlled Trial
Abstract
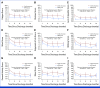
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.We previously reported superior symptom control of electronic patient-reported outcome (ePRO)-based symptom management after lung cancer surgery for up to 1 month postdischarge. Here, we present the long-term results (1-12 months) of this multicenter, randomized trial, where patients were assigned 1:1 to receive postoperative ePRO-based symptom management or usual care daily postsurgery, twice weekly postdischarge until 1 month, and at 3, 6, 9, and 12 months postdischarge. Long-term patient-reported outcomes were assessed with MD Anderson Symptom Inventory-Lung Cancer module. Per-protocol analyses were performed with 55 patients in the ePRO group and 57 in the usual care group. At 12 months postdischarge, the ePRO group reported significantly fewer symptom threshold events (any of the five target symptom scored ≥4; median [IQR], 0 [0-0] v 0 [0-1]; P = .040) than the usual care group. From 1 to 12 months postdischarge, the ePRO group consistently reported significantly lower composite scores for physical interference (estimate, -0.86 [95% CI, -1.32 to -0.39]) and affective interference (estimate, -0.70 [95% CI, -1.14 to -0.26]). Early intensive ePRO-based symptom management after lung cancer surgery reduced symptom burden and improved functional status for up to 1 year postdischarge, supporting its integration into standard care.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures



Comment in
-
Electronic patient-reported outcome-based symptom management, a new era in surgical patient management.J Thorac Dis. 2025 Feb 28;17(2):531-534. doi: 10.21037/jtd-24-1427. Epub 2025 Feb 23. J Thorac Dis. 2025. PMID: 40083483 Free PMC article. No abstract available.
Similar articles
-
Patient-Reported Outcome-Based Symptom Management Versus Usual Care After Lung Cancer Surgery: A Multicenter Randomized Controlled Trial.J Clin Oncol. 2022 Mar 20;40(9):988-996. doi: 10.1200/JCO.21.01344. Epub 2022 Jan 7. J Clin Oncol. 2022. PMID: 34995100 Free PMC article. Clinical Trial.
-
Impact of Remote Symptom Management on Exercise Adherence After Video-Assisted Thoracic Surgery for Lung Cancer in a Tertiary Hospital in China: Protocol for a Prospective Randomized Controlled Trial.JMIR Res Protoc. 2025 Jan 1;14:e60420. doi: 10.2196/60420. JMIR Res Protoc. 2025. PMID: 39610048 Free PMC article.
-
Using patient-reported outcomes to manage postoperative symptoms in patients with lung cancer: protocol for a multicentre, randomised controlled trial.BMJ Open. 2019 Aug 26;9(8):e030041. doi: 10.1136/bmjopen-2019-030041. BMJ Open. 2019. PMID: 31455710 Free PMC article.
-
Behavioural modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation.Health Technol Assess. 2020 Sep;24(46):1-490. doi: 10.3310/hta24460. Health Technol Assess. 2020. PMID: 32975190 Free PMC article.
-
Digital technology for delivering and monitoring exercise programs for people with cystic fibrosis.Cochrane Database Syst Rev. 2023 Jun 9;6(6):CD014605. doi: 10.1002/14651858.CD014605.pub2. Cochrane Database Syst Rev. 2023. PMID: 37294546 Free PMC article. Review.
Cited by
-
Physiological and psychological symptom management based on electronic patient-reported outcomes: the TD-WELLBEING randomized clinical trial.Br J Cancer. 2025 Aug 7. doi: 10.1038/s41416-025-03110-5. Online ahead of print. Br J Cancer. 2025. PMID: 40775446
-
Long-Term Function Recovery Following Upper Versus Lower Lobectomy for Lung Cancer: A Multicenter Longitudinal Cohort Study.Thorac Cancer. 2025 Jan;16(1):e15505. doi: 10.1111/1759-7714.15505. Epub 2024 Dec 13. Thorac Cancer. 2025. PMID: 39670353 Free PMC article.
-
Remote symptom monitoring with patient-reported outcomes and nudges during lung cancer immunotherapy in China (PRO-NET): protocol for a randomised controlled trial.BMJ Open. 2025 Jan 28;15(1):e093374. doi: 10.1136/bmjopen-2024-093374. BMJ Open. 2025. PMID: 39880457 Free PMC article.
-
Patient-centered supportive roadmap: patients and caregivers need to drive their care.Interdiscip Cardiovasc Thorac Surg. 2025 Mar 5;40(3):ivaf033. doi: 10.1093/icvts/ivaf033. Interdiscip Cardiovasc Thorac Surg. 2025. PMID: 40071565 Free PMC article. No abstract available.
-
Effectiveness of symptom monitoring on electronic patient-reported outcomes (ePROs) among patients with lung cancer: a systematic review and meta-analysis.NPJ Digit Med. 2025 Jul 3;8(1):399. doi: 10.1038/s41746-025-01812-x. NPJ Digit Med. 2025. PMID: 40604235 Free PMC article.
References
-
- Hirpara DH, Coburn NG, Darling GE, et al. : Symptom assessment following surgery for lung cancer: A Canadian population-based retrospective cohort study. Ann Surg 277:e428-e438, 2023 - PubMed
-
- Smith AB, Basch E: Role of patient-reported outcomes in postsurgical monitoring in oncology. J Oncol Pract 13:535-538, 2017 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

