Astrocyte-secreted neurocan controls inhibitory synapse formation and function
- PMID: 38574730
- PMCID: PMC11098688
- DOI: 10.1016/j.neuron.2024.03.007
Astrocyte-secreted neurocan controls inhibitory synapse formation and function
Abstract
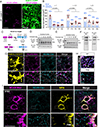
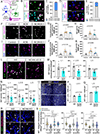
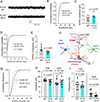
Astrocytes strongly promote the formation and maturation of synapses by secreted proteins. Several astrocyte-secreted synaptogenic proteins controlling excitatory synapse development were identified; however, those that induce inhibitory synaptogenesis remain elusive. Here, we identify neurocan as an astrocyte-secreted inhibitory synaptogenic protein. After secretion from astrocytes, neurocan is cleaved into N- and C-terminal fragments. We found that these fragments have distinct localizations in the extracellular matrix. The neurocan C-terminal fragment localizes to synapses and controls cortical inhibitory synapse formation and function. Neurocan knockout mice lacking the whole protein or only its C-terminal synaptogenic domain have reduced inhibitory synapse numbers and function. Through super-resolution microscopy, in vivo proximity labeling by secreted TurboID, and astrocyte-specific rescue approaches, we discovered that the synaptogenic domain of neurocan localizes to somatostatin-positive inhibitory synapses and strongly regulates their formation. Together, our results unveil a mechanism through which astrocytes control circuit-specific inhibitory synapse development in the mammalian brain.
Keywords: astrocytes; chondroitin sulfate proteoglycans; extracellular matrix; inhibitory synaptogenesis; interneurons; in vivo TurboID; lecticans; neurocan; perineuronal nets; somatostatin.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Update of
-
Astrocyte-Secreted Neurocan Controls Inhibitory Synapse Formation and Function.bioRxiv [Preprint]. 2023 Apr 3:2023.04.03.535448. doi: 10.1101/2023.04.03.535448. bioRxiv. 2023. Update in: Neuron. 2024 May 15;112(10):1657-1675.e10. doi: 10.1016/j.neuron.2024.03.007. PMID: 37066164 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

