Fast and Accurate Disulfide Bridge Detection
- PMID: 38574859
- PMCID: PMC11067345
- DOI: 10.1016/j.mcpro.2024.100759
Fast and Accurate Disulfide Bridge Detection
Abstract
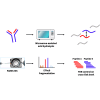
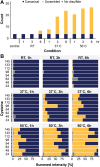
Recombinant expression of proteins, propelled by therapeutic antibodies, has evolved into a multibillion dollar industry. Essential here is the quality control assessment of critical attributes, such as sequence fidelity, proper folding, and posttranslational modifications. Errors can lead to diminished bioactivity and, in the context of therapeutic proteins, an elevated risk for immunogenicity. Over the years, many techniques were developed and applied to validate proteins in a standardized and high-throughput fashion. One parameter has, however, so far been challenging to assess. Disulfide bridges, covalent bonds linking two cysteine residues, assist in the correct folding and stability of proteins and thus have a major influence on their efficacy. Mass spectrometry promises to be an optimal technique to uncover them in a fast and accurate fashion. In this work, we present a unique combination of sample preparation, data acquisition, and analysis facilitating the rapid and accurate assessment of disulfide bridges in purified proteins. Through microwave-assisted acid hydrolysis, the proteins are digested rapidly and artifact-free into peptides, with a substantial degree of overlap over the sequence. The nonspecific nature of this procedure, however, introduces chemical background, which is efficiently removed by integrating ion mobility preceding the mass spectrometric measurement. The nonspecific nature of the digestion step additionally necessitates new developments in data analysis, for which we extended the XlinkX node in Proteome Discoverer to efficiently process the data and ensure correctness through effective false discovery rate correction. The entire workflow can be completed within 1 h, allowing for high-throughput, high-accuracy disulfide mapping.
Keywords: EThcD; FAIMS; MAAH; XlinkX/PD; disulfide bridge.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest Y. H., Y. S., and R. V. are employees of Thermo Fisher Scientific, the manufacturer of the Orbitrap and the Proteome Discoverer platforms used in this work. The other authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

