Phenotypical, genotypical and pathological characterization of the moonwalker mouse, a model of ataxia
- PMID: 38575093
- PMCID: PMC11089908
- DOI: 10.1016/j.nbd.2024.106492
Phenotypical, genotypical and pathological characterization of the moonwalker mouse, a model of ataxia
Abstract
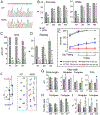
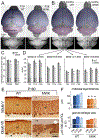
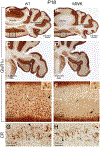
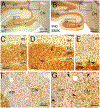
We performed a comprehensive study of the morphological, functional, and genetic features of moonwalker (MWK) mice, a mouse model of spinocerebellar ataxia caused by a gain of function of the TRPC3 channel. These mice show numerous behavioral symptoms including tremor, altered gait, circling behavior, impaired motor coordination, impaired motor learning and decreased limb strength. Cerebellar pathology is characterized by early and almost complete loss of unipolar brush cells as well as slowly progressive, moderate loss of Purkinje cell (PCs). Structural damage also includes loss of synaptic contacts from parallel fibers, swollen ER structures, and degenerating axons. Interestingly, no obvious correlation was observed between PC loss and severity of the symptoms, as the phenotype stabilizes around 2 months of age, while the cerebellar pathology is progressive. This is probably due to the fact that PC function is severely impaired much earlier than the appearance of PC loss. Indeed, PC firing is already impaired in 3 weeks old mice. An interesting feature of the MWK pathology that still remains to be explained consists in a strong lobule selectivity of the PC loss, which is puzzling considering that TRPC is expressed in every PC. Intriguingly, genetic analysis of MWK cerebella shows, among other alterations, changes in the expression of both apoptosis inducing and resistance factors possibly suggesting that damaged PCs initiate specific cellular pathways that protect them from overt cell loss.
Keywords: Apoptosis; Calcium; Cerebellum; Endoplasmic reticulum; Neurodegeneration; Purkinje cells; Spinocerebellar ataxia; TRPC3; Unipolar brush cell.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no competing financial interest.
Figures








Similar articles
-
A novel early onset spinocerebellar ataxia 13 BAC mouse model with cerebellar atrophy, tremor, and ataxic gait.Exp Anim. 2025 Jul 11;74(3):362-374. doi: 10.1538/expanim.24-0118. Epub 2025 Mar 20. Exp Anim. 2025. PMID: 40128944 Free PMC article.
-
Cerebellar Heterogeneity and Selective vulnerability in Spinocerebellar Ataxia Type 1 (SCA1).Neurobiol Dis. 2024 Jul;197:106530. doi: 10.1016/j.nbd.2024.106530. Epub 2024 May 14. Neurobiol Dis. 2024. PMID: 38750673 Free PMC article.
-
Dysregulation of zebrin-II cell subtypes in the cerebellum is a shared feature across polyglutamine ataxia mouse models and patients.Sci Transl Med. 2024 Nov 6;16(772):eadn5449. doi: 10.1126/scitranslmed.adn5449. Epub 2024 Nov 6. Sci Transl Med. 2024. PMID: 39504355 Free PMC article.
-
The Moonwalker mouse: new insights into TRPC3 function, cerebellar development, and ataxia.Cerebellum. 2014 Oct;13(5):628-36. doi: 10.1007/s12311-014-0564-5. Cerebellum. 2014. PMID: 24797279 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

