Effectiveness and durability of mRNA-1273 BA.4/BA.5 bivalent vaccine (mRNA-1273.222) against SARS-CoV-2 BA.4/BA.5 and XBB sublineages
- PMID: 38575149
- PMCID: PMC10996830
- DOI: 10.1080/21645515.2024.2335052
Effectiveness and durability of mRNA-1273 BA.4/BA.5 bivalent vaccine (mRNA-1273.222) against SARS-CoV-2 BA.4/BA.5 and XBB sublineages
Abstract
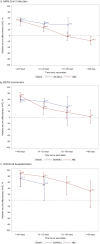
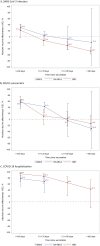
Emerging SARS-CoV-2 sublineages continue to cause serious COVID-19 disease, but most individuals have not received any COVID-19 vaccine for >1 year. Assessment of long-term effectiveness of bivalent COVID-19 vaccines against circulating sublineages is important to inform the potential need for vaccination with updated vaccines. In this test-negative study at Kaiser Permanente Southern California, sequencing-confirmed BA.4/BA.5- or XBB-related SARS-CoV-2-positive cases (September 1, 2022 to June 30, 2023), were matched 1:3 to SARS-CoV-2-negative controls. We assessed mRNA-1273 bivalent relative (rVE) and absolute vaccine effectiveness (VE) compared to ≥2 or 0 doses of original monovalent vaccine, respectively. The rVE analysis included 20,966 cases and 62,898 controls. rVE (95%CI) against BA.4/BA.5 at 14-60 days and 121-180 days was 52.7% (46.9-57.8%) and 35.5% (-2.8-59.5%) for infection, and 59.3% (49.7-67.0%) and 33.2% (-28.2-68.0%) for Emergency Department/Urgent Care (ED/UC) encounters. For BA.4/BA.5-related hospitalizations, rVE was 71.3% (44.9-85.1%) and 52.0% (-1.2-77.3%) at 14-60 days and 61-120 days, respectively. rVE against XBB at 14-60 days and 121-180 days was 48.8% (33.4-60.7%) and -3.9% (-18.1-11.3%) for infection, 70.7% (52.4-82.0%) and 15.7% (-6.0-33.2%) for ED/UC encounters, and 87.9% (43.8-97.4%) and 57.1% (17.0-77.8%) for hospitalization. VE and subgroup analyses (age, immunocompromised status, previous SARS-CoV-2 infection) results were similar to rVE analyses. rVE of mRNA-1273 bivalent vaccine against BA.4/BA.5 and XBB infections, ED/UC encounters, and hospitalizations waned over time. Periodic revaccination with vaccines targeting emerging variants may be important in reducing COVID-19 morbidity and mortality.
Keywords: BA.4; BA.4/BA.5; BA.5; Bivalent; COVID-19; XBB; durability; mRNA-1273; omicron; vaccine effectiveness.
Conflict of interest statement
BKA, LQ, LSS, SQ, JET, GSL, JHK, YL, RB, JS, SKC, HST, MA, and HFT are employees of Kaiser Permanente Southern California (KPSC), contracted by Moderna, Inc. to conduct this study. KJB is an adjunct investigator at (KPSC). AF was an employee of (KPSC); currently an employee of SimulStat. MAM, EJA, CKZ, and TS are employees of and shareholders in Moderna, Inc. CAT was an employee of and a shareholder in Moderna Inc at the time of protocol development; currently an employee of AstraZeneca. BKA received funding from GlaxoSmithKline, Dynavax, Genentech, and Moderna unrelated to this manuscript. LQ received funding from GlaxoSmithKline, Dynavax, and Moderna unrelated to this manuscript. LSS received funding from GlaxoSmithKline, Dynavax, and Moderna unrelated to this manuscript. KJB received funding from GlaxoSmithKline, Dynavax, and Pfizer unrelated to this manuscript. SQ received funding from Dynavax unrelated to this manuscript. JET received funding from Pfizer and Moderna unrelated to this manuscript. GSL, JHK, and HFT received funding from GlaxoSmithKline and Moderna unrelated to this manuscript. HFT served on advisory boards for Janssen and Pfizer. AF received funding from Pfizer, GlaxoSmithKline, Gilead, and Moderna unrelated to this manuscript. YL received funding from GlaxoSmithKline, Pfizer, and Moderna unrelated to this manuscript. RB received funding from GlaxoSmithKline unrelated to this manuscript. JS received funding from Pfizer, Sanofi, and Intercept unrelated to this manuscript. SKC received funding from Pfizer, Bayer AG, and Pancreatic Cancer Action Network unrelated to this manuscript. HST received funding from GlaxoSmithKline, Pfizer, ALK, and Wellcome unrelated to this manuscript. MA received funding from Pfizer unrelated to this manuscript.
Figures


References
-
- Centers for Disease Control and Prevention . COVID data tracker: trends. [accessed 2023 Oct 7]. https://covid.cdc.gov/covid-data-tracker/#datatracker-home.
-
- U.S. Food and Drug Administration . Coronavirus (COVID-19) update: FDA authorizes moderna, pfizer-BioNTech bivalent COVID-19 vaccines for use as a booster dose. 2022. [accessed 2023 Jun 17]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19....
-
- U.S. Food and Drug Administration . Coronavirus (COVID-19) update: FDA authorizes changes to simplify use of bivalent mRNA COVID-19 vaccines. 2023. [accessed 2023 Jun 17]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19....
-
- Tenforde MW, Weber ZA, Natarajan K, Klein NP, Kharbanda AB, Stenehjem E, Embi PJ, Reese SE, Naleway AL, Grannis SJ, et al. Early estimates of bivalent mRNA vaccine effectiveness in preventing COVID-19–associated Emergency Department or urgent care encounters and hospitalizations among immunocompetent adults — VISION network, Nine States, September–November 2022. MMWR Morb Mortal Wkly Rep. 2023. Mar 17;71(12):1637–46. doi:10.15585/mmwr.mm7153a1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
