Benralizumab in severe eosinophilic asthma by previous biologic use and key clinical subgroups: real-world XALOC-1 programme
- PMID: 38575162
- PMCID: PMC11237372
- DOI: 10.1183/13993003.01521-2023
Benralizumab in severe eosinophilic asthma by previous biologic use and key clinical subgroups: real-world XALOC-1 programme
Abstract
Background: Pivotal phase 3 trials and real-world studies have demonstrated benralizumab's overall efficacy and safety in severe eosinophilic asthma (SEA). Additional large-cohort data are needed to confirm its real-world effectiveness in SEA according to previous biologic use and key baseline characteristics important for treatment selection.
Methods: XALOC-1 is a large, multinational, retrospective, observational, real-world study programme of benralizumab in adults with SEA. This 48-week integrated analysis assessed annualised exacerbation rate (AER), maintenance oral corticosteroid (mOCS) use, asthma symptom control and lung function during a 12-month baseline period and up to 48 weeks after benralizumab initiation. Subgroup analyses were based on previous biologic use and key baseline clinical characteristics (mOCS use, blood eosinophil count, exacerbation history, age at asthma diagnosis, fractional exhaled nitric oxide level and presence of atopy and chronic rhinosinusitis with nasal polyps).
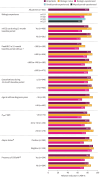
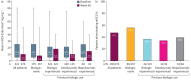
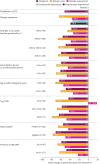
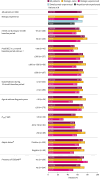
Results: Out of 1002 patients analysed, 380 were biologic-experienced. At week 48, 71.3% were exacerbation-free (versus 17.2% at baseline); relative reduction in AER was 82.7% overall and 72.9% in biologic-experienced patients; rates were maintained across all key clinical characteristic subgroups. Of patients using mOCS at baseline (n=274), 47.4% (130 out of 274) eliminated their use by week 48; the mean reduction from baseline in daily dose was 51.2% and, notably, 34.9% in biologic-experienced patients (n=115). Clinically significant improvements in asthma symptom control and lung function were observed.
Conclusion: In this large, real-world programme, SEA patients treated with benralizumab had substantial improvements in clinical outcomes irrespective of previous biologic use and key clinical characteristics important to therapeutic decision-making in clinical practice.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: D.J. Jackson has received consultancy fees and speakers’ fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline and Sanofi Regeneron, and research grants from AstraZeneca. G. Pelaia has received lecture fees and advisory board fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Guidotti, Insmed, Lusofarmaco, Menarini, Neopharmed Gentili, Novartis, Sanofi and Zambon. B. Emmanuel, T.N. Tran, D. Cohen, V.H. Shih, A. Shavit, M. Watt, S. Kayaniyil, M. Pardal, D. Arbetter and A.P.J. Rabe are employees of, and own stock in, AstraZeneca. S. Boarino and J. Nuevo are employees of AstraZeneca. R. Katial (currently of National Jewish Health and University of Colorado Denver, Denver, CO, USA) and E. Garcia-Gil (currently of Almirall, Barcelona, Spain) were employees of AstraZeneca at the time the study was conducted. C. Chaves Loureiro has received consultancy fees and speakers’ fees from AstraZeneca, Chiesi, GlaxoSmithKline, Sanofi Regeneron and Teva, and research grants from GlaxoSmithKline. A. Padilla-Galo reports grants, personal fees and non-financial support from AstraZeneca and Sanofi, personal fees, and non-financial support from Chiesi, GlaxoSmithKline, Novartis and Teva, and personal fees from ALK, Bial and FAES, outside the submitted work. P. Nair reports that in the past two years, his institution received grant support from AstraZeneca, Cyclomedica, Equillium, Foresee, Genentech, Sanofi and Teva; he has also received honoraria from Arrowhead, AstraZeneca, CSL Behring, GlaxoSmithKline and Sanofi.
Figures

Comment in
-
Real world evidence in asthma: what to expect beyond randomised controlled trials?Eur Respir J. 2024 Jul 11;64(1):2400716. doi: 10.1183/13993003.00716-2024. Print 2024 Jul. Eur Respir J. 2024. PMID: 38991724 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
