Transient Seizure Clusters and Epileptiform Activity Following Widespread Bilateral Hippocampal Interneuron Ablation
- PMID: 38575351
- PMCID: PMC11036118
- DOI: 10.1523/ENEURO.0317-23.2024
Transient Seizure Clusters and Epileptiform Activity Following Widespread Bilateral Hippocampal Interneuron Ablation
Abstract
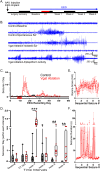
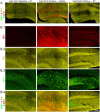
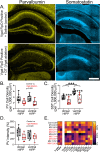
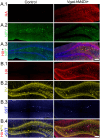
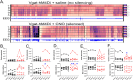
Interneuron loss is a prominent feature of temporal lobe epilepsy in both animals and humans and is hypothesized to be critical for epileptogenesis. As loss occurs concurrently with numerous other potentially proepileptogenic changes, however, the impact of interneuron loss in isolation remains unclear. For the present study, we developed an intersectional genetic approach to induce bilateral diphtheria toxin-mediated deletion of Vgat-expressing interneurons from dorsal and ventral hippocampus. In a separate group of mice, the same population was targeted for transient neuronal silencing with DREADDs. Interneuron ablation produced dramatic seizure clusters and persistent epileptiform activity. Surprisingly, after 1 week seizure activity declined precipitously and persistent epileptiform activity disappeared. Occasional seizures (≈1/day) persisted to the end of the experiment at 4 weeks. In contrast to the dramatic impact of interneuron ablation, transient silencing produced large numbers of interictal spikes, a significant but modest increase in seizure occurrence and changes in EEG frequency band power. Taken together, findings suggest that the hippocampus regains relative homeostasis-with occasional breakthrough seizures-in the face of an extensive and abrupt loss of interneurons.
Keywords: DREADDs; Vgat; diphtheria toxin; epileptogenesis; neuropeptide Y; parvalbumin.
Copyright © 2024 Dusing et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
